- Information by Map Unit
- Information by Topic
- Documents in SoLo
Links to other related sites
- About SoLo
-
- Mission
- Disclaimer
- Authors (complete list)
Fire affects nutrient cycling and the physical, chemical, and biological properties of soils occupied by western-montane forests. Combustion of litter and soil organic matter (OM) increases the availability of some nutrients, although others are volatilized (for example, N, P, S). Soil OM loss also affects cation exchange capacity, organic chelation, aggregate stability, macro pore space, infiltration, and soil microorganisms. Nitrogen replenishment must be emphasized when prescribed burning programs are planned or during rehabilitation following wildfires.
Fire significantly affects soil properties because organic matter (OM) located on, or near, the soil surface is rapidly combusted. The changes in OM, in turn, affect several chemical, physical, and microbiological properties of the underlying soil. Although some nutrients are volatilized and lost, most nutrients are made more available. Fire acts as a rapid mineralizing agent (St. John and Rundel 1976) that releases nutrients instantaneously as contrasted to natural decomposition processes, which may require years or, in some cases, decades.
The objectives of this paper are to (1) review the importance of OM and plant nutrients in the soil, (2) describe changes in OM during combustion, (3) characterize several soil physical, chemical, and biological temperature thresholds, and (4) identify and discuss the more important fire-related changes occurring in soils that affect postfire management.
Organic matter in western-montane forest soils is concentrated on, or near, the soil surface and is made up of six easily recognized components: (1) the litter layer, consisting of recognizable plant litter; (2) the duff layer, composed of partially decomposed, but recognizable, plant litter; (3) the humus layer, consisting of extensively decayed and disintegrated organic materials, which are sometimes mixed with mineral soil; (4) decayed wood, consisting of the residual lignin matrix from decaying woody material that is on the soil surface or has been buried by the forest floor; (5) charcoal, or extensively charred wood mixed into the mineral soil; and (6) the upper mineral soil horizon (A horizon) of the underlying mineral soil (Harvey 1982). Soil OM plays an important role in the physical, chemical, and biological properties of the soil and, as such, contributes to overall soil and site productivity.
Organic matter acts as the primary reservoir for several nutrients and, therefore, is the source for most of the available phosphorus (P) and sulfur (S), and virtually all of the available nitrogen (N). Soil OM's role in N storage is especially important in forests because their continued high productivity depends, to a large extent, on large supplies of available N. Nutrients stored in OM are released slowly during decomposition, providing an efficient, steady source of nutrients that keeps leaching losses at low levels.
Soil OM and humus also provide chemically active cation exchange sites that retain many of the important cations (for example, NH4+, K+, Ca++). It has been estimated that soil OM can provide over 50 percent of the cation exchange capacity of some forest soils. It also is an active chelating agent that retains many of the metals.
Organic matter serves as a powerful aggregating agent and, as such, plays an important role in creating and maintaining a well-aggregated soil. Soil aggregation improves soil structure that creates macro pore space, and improves soil aeration. Aggregate soils also have higher infiltration rates than nonaggregated soils containing less OM.
The welfare of soil microorganisms also depends on OM because it provides both a suitable environment and C compounds that serve as an energy source for soil microorganisms. Both of these functions are critical for maintaining the nutritional quality and moisture-holding capacity of forest soils (Harvey and others 1987).
Nitrogen is an extremely important nutrient because it is the one that is most likely to limit tree growth in forests and other wildland ecosystems (Maars and others 1983). Because of this inherent limitation, significant losses of N during a fire could adversely affect long-term site productivity in many wildland ecosystems, particularly if N replenishment mechanisms are not provided for during postfire management.
Nitrogen contained in unburned forest litter and soil is released solely by biological processes and is referred to as being regulated by "biochemical cycling" (McGill and Cole 1981). Because of the close relationship between carbon (C) and N, C:N ratios play an important role in regulating the decomposition rate of OM and, as a result, control the rate at which N and other nutrients are released and cycled (Turner 1977).
The role of S in ecosystem productivity is not well understood, although its fluctuations in the soil appear to parallel that of inorganic N. Sulfur is considered the second most limiting nutrient in some coastal forest soils of the Pacific Northwest, particularly when forest stands are fertilized with N (Barnett 1989).
Phosphorus also has been reported to be limiting in some forest ecosystems. Deficiencies of P are most likely to appear in P-fixing soils (Vlamis and others 1955) or in conjunction with N fertilization applications (Heilman and Gessel 1963). Phosphorus uptake and availability to plants also appear to be highly dependent on the interrelationship between mycorrhizae and OM rather than being a simple absorption from the soil solution (Trappe and Bollen 1979). Deficiencies of the major cations (Ca, Mg, and K) have not been reported for most wildland soils. The balance of these cations determines base saturation, which plays an important role in controlling pH regimes in soils.
During combustion, soil OM undergoes a series of physical and chemical transformations (Chandler and others 1983). Initially, the free moisture is vaporized as soon as the temperature approaches 100°C. Lignin and hemicellulose begin to degrade at temperatures between 130 and 190°C. Reactions occurring at temperatures below 200°C are endothermic (reactions that require the absorption of heat). Decomposition of lignin and hemicellulose becomes rapid at 200°C with cellulose undergoing chemical dehydration at 280°C. About 35 percent of the total weight loss occurs before soil OM reaches 280°C. Once soil temperatures exceed 280°C, exothermic reactions (those reactions that produce heat) predominate and OM is ignited. When the surface temperature of soil OM reaches 500 to 600°C, glowing combustion occurs if oxygen is not excluded from the char surface. Flaming then occurs and boosts temperatures from 800 to 1,500°C. Above 1,000°C carbon (C) is consumed at the surface as rapidly as char is produced.
Most of the thermal energy released during the combustion of aboveground fuels is lost upward into the atmosphere (DeBano 1974). However, a lesser but significant amount is radiated downward and is absorbed by the surface litter when present, or by other organic layers, depending on the amount and configuration of the OM deposited on the soil surface. The radiated heat can produce secondary combustion of the litter, duff, and, in some cases, the soil humus layer.
The amounts of litter, duff, and humus combusted depend on the duration and intensity of the heat flux reaching the litter layer. The combustion of aboveground fuels, and more important, duff, may heat the mineral soil surface significantly, and as a result substantial amounts of heat can be transferred downward into the soil by conduction, convection, and by vaporization and condensation. Thus, soil temperatures generated during fires vary considerably, depending on the fuel load and the burning conditions (DeBano 1989). If a large amount of fuel is present, soil temperatures can remain high for several hours and would be expected to produce large changes in soil chemical, physical, and biological properties. In contrast, soil temperatures produced during low-intensity fires used for fuel reduction may not produce appreciable changes in the soil OM.
The spatial distribution of soil properties within a soil profile determine, to a large extent, the magnitude of change occurring in a particular soil property during a fire. For example, those soil properties located on, or near, the soil surface are more likely to be changed by fire because they are directly exposed to surface heating. As a result, organic material and related soil properties are more likely changed by radiated energy than other soil properties, such as clay content, which is often concentrated in subsurface layers where it is insulated from surface heating.
The sensitivity of a particular soil property to heating is also important. In general, changes in soil chemical properties are directly related to the changes in OM described earlier. However, some soil physical properties are also dependent on soil OM, while others are not (for example, clay content). Soil microorganisms are probably most sensitive to soil heating because they are living organisms that have relatively low lethal-temperature thresholds.
Nutrients contained in fuel and soil OM are cycled by biological decomposition processes in environments where temperatures rarely approach 38°C and sufficient moisture is available for sustaining active microbial activity. Under these mild conditions, soil microorganisms decompose OM and slowly release many of the essential nutrients over time. In contrast, during a fire the nutrients stored in fuels and soil OM are subjected to severe heating and, as a result, undergo various irreversible transformations during combustion. However, the responses of individual nutrients differ and each has its inherent temperature threshold. Threshold temperatures are defined as those temperatures where volatilization of a nutrient occurs. For discussion purposes, these thresholds can be divided into three general nutrient categories: sensitive, moderately sensitive, and relatively insensitive. Nitrogen (Hosking 1938) and S (Tiedemann 1987) are considered sensitive because they have thresholds as low as 200 to 375°C, respectively. Potassium (K) and P are moderately sensitive, having threshold temperatures of 774°C (Raison and others 1985). Magnesium (Mg), calcium (Ca), and manganese (Mn) are relatively insensitive, with high threshold temperatures of 1,107°C, 1,484°C, and 1,962°C, respectively. Because the threshold temperatures of N, P, K, and S are lower than the flaming temperatures of woody fuels (1,100°C and, except for P, lower than glowing combustion temperatures (650°C), these nutrients are readily volatilized from OM during combustion.
Nutrient Losses—Because some nutrients, such as N, P, and S, have low temperature thresholds and are easily volatilized, it is important to consider their losses in more detail. Nitrogen will be used to illustrate nutrient losses by volatilization because it is the nutrient that is most likely to be limiting in forest ecosystems.
The amount of total N volatilized during combustion has been reported to be directly proportional to the amount of OM combusted (Raison and others 1985). Most of this volatilized N (up to 99 percent) reverts to N2 (DeBell and Ralston 1970). This relationship may not hold at lower temperatures (Grier 1975), because OM can decompose without volatilizing N; therefore, N loss is not proportional to the loss of OM. The N that is not volatilized remains on the site either in uncombusted fuels or as highly available ammonium-N (NH4-N) in the soil.
Phosphorus responds differently, and only about 60 percent of the total P is lost by nonparticulate transfer when fuels are totally consumed (Raison and others 1985). As a result, substantial amounts of highly available P can be found in the ash and on the soil surface immediately following fire. Percentage loss of S by volatilization is intermediate to N and P (Tiedemann 1987), and burning has been reported to remove 20 to 40 percent of the S in aboveground biomass (Barnett 1989).
Nutrient Availability—Most changes in nutrient availability result from two different processes: (1) in situ changes, and (2) translocation of organic substances downward into the soil.
Heating the underlying mineral soil directly affects nutrients contained in the soil OM (in situ changes). However, the responses of the different nutrients to heating indicate little change is likely to occur more than 4 to 5 cm below the soil surface, unless a very intense, long-duration fire occurs (for example, in piles of logs).
More important, nutrient availability (particularly N) in the soil can be increased by the translocation of nutrients downward into the soil during a fire. This occurs because steep temperature gradients are produced in the upper soil layers during the combustion of the litter and humus on the soil surface. During combustion, surface soil temperatures may exceed 1,000°C. Poor heat conduction by the soil results in temperatures of 200°C or less within 5 cm of the soil surface. As a result, some of the vaporized OM and ammonium-rich nitrogenous compounds released during combustion are transferred downward where they condense in the cooler underlying soil (DeBano and others 1976).
Although large amounts of total N are lost during the combustion of plants and litter, available NH4-N is usually higher in the underlying soil following a fire because of the transfer mechanism (DeBano and others 1979). The increase in N availability (as NH4-N) observed immediately following a fire appears related to the soil temperatures reached. For example, under an extremely hot fire most of the soil N is probably volatilized, particularly on or near the soil surface, and only small amounts are transferred downward in the soil. In contrast, under cooler soil-heating regimes, substantial amounts of NH4-N can be found in the ash and underlying soil. Therefore, depending on the severity and duration of the fire, concentrations of NH4-N may increase, decrease, or remain unchanged.
Phosphorus does not appear to be translocated downward in the soil profile as readily as N compounds. As a result, P increases mainly in the ash and on, or near, the soil surface (DeBano 1989; DeBano and Klopatek 1988).
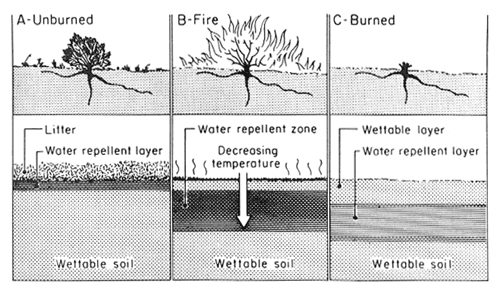
Soil physical properties that are dependent on OM (for example, soil structure, pore space, aggregation) are all affected by heating during a fire. Other soil physical properties, such as clay content, are not readily affected, except on the immediate soil surface during a very intense fire. An important physical property affected by fire, one that regulates the hydrology of a soil, is water repellency (DeBano 1981). During fires, OM in the litter and upper mineral soil layers is volatilized (fig. 1A). Most of the volatilized OM is lost upward in the smoke, but a small amount moves downward along steep temperature gradients in the upper 5 cm of the soil and condenses to form a water-repellent layer that impedes infiltration (fig. 1B,C). The degree of water repellency formed depends on the steepness of temperature gradients near the soil surface, soil water content, and soil physical properties. For example, coarse-textured soils are more susceptible to heat- induced water repellency than fine-textured clay soils. The formation of this water-repellent layer, along with the loss of protective plant cover, increases surface runoff and erosion during the first rains following burning. A reduction in infiltration by a water-repellent layer can lead to extensive rill erosion on burned watersheds (Wells 1981).
Figure 1—Soil-water repellency as altered by fire: (A) before fire, hydrophobic substances accumulate in the litter layer and mineral soil immediately beneath; (B) fire burns the vegetation and litter layer, causing hydrophobic substances to move downward along temperature gradients; (C) after fire, a water-repellent layer is present below and parallel to the soil surface on the burned area (DeBano 1981). [view larger image - 76K] [Text description of this figure]
Soil heating directly affects microorganisms by either killing them directly or altering their reproductive capabilities. Indirectly, soil heating alters OM (energy source) and increases nutrient availability, thereby affecting subsequent microbial growth. Although the relationship between soil heating and soil microbial populations is complex, it appears that duration of heating, maximum temperatures, and soil water all affect microbial responses (Dunn and others 1985). Microbial groups differ significantly in their sensitivity to temperature and nitrifying bacteria appear to be particularly sensitive to soil heating (Dunn and others 1985). Physiologically active populations of microorganisms in moist soil are more sensitive than dormant populations in dry soil.
Another important group of soil microorganisms that are particularly sensitive to soil heating during a fire are endo- and ectomycorrhizae. Because most ectomycor-rhizae are concentrated in the OM on or near the soil surface, the loss of shallow organic layers may be at least partially responsible for the reported fire-related reductions in ectomycorrhizal activity of western conifers (Harvey and others 1989). Likewise, ectomycorrhizae (for example, vesicular-arbuscular mycorrhizae [VAM]) in pinyon-juniper woodlands were also reported to be affected by soil heating (Klopatek and others 1988). This decrease in VAM colonization may be an important factor affecting the long-term productivity of forest ecosystems.
Postfire management must play a key role in the development and implementation of any prescribed burning program or rehabilitation project following wildfires. Many of the general relationships between fire severity, soil heating, OM, and associated changes in soil properties discussed here have direct application when one assesses fire effects in soils in the western-montane forest environments and, thus, must be considered when post- fire management guidelines are formulated.
Although little can be done to control OM loss during wildfires, every opportunity must be taken to revegetate the site so that organic litter can again be restored on the site as quickly as possible. When one plans prescribed fires, more opportunities are available for maintaining an acceptable level of OM than occur following wildfires. For example, burning prescriptions can be designed to avoid burns that consume large amounts of surface litter and soil humus. Likewise, the total combustion of large woody debris on the soil surface (logs, etc.) during prescribed burning may not be a desirable practice. Repeated use of fire at frequent intervals probably should be avoided on relatively infertile sites where OM production is inherently low (for example, south-facing slopes), although it can play an important role in nutrient cycling in those ecosystems that experience frequent low-intensity fires (such as, ponderosa pine forests).
Because N is such an important nutrient in ecosystems and large losses are likely to occur by volatilization during the combustion of OM, special consideration must be given to both its loss and replenishment when planning burning programs. Important considerations to keep in mind when evaluating the effect of fire on N cycling are: size of the total N pool, type of fuel consumed, severity of the burn, and, more important, the mechanisms responsible for replacing N lost by volatilization.
Because of the large N losses, mechanisms for N replenishment in the soil must be considered an important part of postfire management. Nitrogen additions to the soil can come from several sources including: (1) small amounts of N present in precipitation and dust; (2) con- version or "fixation" of atmospheric N2 gas into usable forms by soil- and root-inhabiting microorganisms; and (3) mineral or organic fertilizers. Atmospheric inputs are usually small in relation to other inputs and typically range from 1 to 4 kg/ha/yr in the Pacific Northwest (Barnett 1989). Atmospheric N fixed by microorganisms ranges from 32 to 320 kg/ha/yr in fully stocked red alder (Alnus rubra) (Barnett 1989) to 0.1 to 1.3 kg/ha/yr by free-living N-fixers (Jurgensen and others 1979). Harvey and others (1989) found that more than one-third of the N-fixing capacity of some forest soils can be provided by microorganisms responsible for decaying wood on the surface and in the soil profile; thus, management of woody residues within a fire prescription may be an important dimension of N management in a fire environment.
Both wild and prescribed fires occur frequently in western-montane forests. These fires dramatically affect the nutrient cycling and the physical, chemical, and biological properties of the underlying soil. Substantial amounts of C, N, S, and P can also be lost to the atmosphere by volatilization during the combustion of litter, duff, and soil OM.
Because N is such an important nutrient in these ecosystems, the replenishment of N lost by volatilization during a fire must receive special consideration when burning programs are being planned or during rehabilitation following wildfires. Treatments interfering with the establishment of postfire N-fixing plants should be avoided, particularly on infertile soils having low site potentials. Woody residue management also appears to be an important factor in N fixation and may require special attention when fire prescriptions are being developed.
Burning increases the availability of most plant nutrients. Although some nutrients are volatilized during combustion, available NH4-N and P increase substantially during burning. High concentrations of available plant nutrients on the soil surface immediately following fire may negate the advantage of fertilizing for at least 1 year after burning.
In the final analysis, fire plays an important role in the management of ecosystems, not only in the western-montane forests, but throughout the world. Although fire can dramatically affect soil properties and cycling, its effects can be mitigated by development of informed burning prescriptions or by careful selection of rehabilitation treatments following wildfires. However, careful planning is necessary to assure the sustained long-term productivity of these ecosystems is not adversely affected by fire-related changes in soils.
Barnett, Dwight. 1989. Fire effects on Coast Range soils of Oregon and Washington and management implications: a state-of-knowledge review. R-6 Soils Tech. Rep. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 66 p.
Chandler, Craig; Cheney, Phillip; Thomas, Philip; Trabaud, Louis; Williams, Dave. 1983. Fire in forestry. Vol. 1: forest fire behavior and effects. New York: John Wiley & Sons. 450 p.
DeBano, L. F. 1974. Chaparral soils. In: Rosenthal, M., ed. Living with the chaparral: symposium proceedings; 1973 March 30-31; Riverside, CA. San Francisco, CA: A Sierra Club Special Publication: 19-26.
DeBano, L. F. 1981. Water repellent soils: a state-of-the-art. Gen. Tech. Rep. PSW-46. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 21 p.
DeBano, Leonard F. 1989. Effects of fire on chaparral soils in Arizona and California and postfire management implications. In: Berg, N., ed. Fire and watershed management: symposium proceedings; 1988 October 16-28; Sacramento, CA. Gen. Tech. Rep. PSW-109. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 55-62.
DeBano, Leonard F.; Eberlein, Gary E.; Dunn, Paul H. 1979. Effects of burning on chaparral soils: I. Soil nitrogen. Soil Science Society of America Journal. 43: 504-509.
DeBano, Leonard F.; Klopatek, Jeffrey M. 1988. Phosphorus dynamics of pinyon-juniper soils following simulated burning. Soil Science Society of America Journal. 52: 271-277.
DeBano, L. F.; Savage, S. M.; Hamilton, D. A. 1976. The transfer of heat and hydrophobic substances during burning. Soil Science Society of America Journal. 40: 779-782.
DeBell, D. S.; Ralston, C. W. 1970. Release of nitrogen by burning light forest fuels. Soil Science Society of America Proceedings. 34: 936-938.
Dunn, Paul H.; Barro, Susan C.; Poth, Mark. 1985. Soil moisture affects survival of microorganisms in heated chaparral soil. Soil Biology and Biochemistry. 17: 143-148.
Grier, C. C. 1975. Wildfire effects on nutrient distribution and leaching in a coniferous ecosystem. Canadian Journal of Forest Research. 5: 559-607.
Harvey, Alan E. 1982. The importance of residual organic debris in site preparation and amelioration for reforestation. In: Baumgartner, David M., ed. Site preparation and fuels management on steep terrain: symposium proceedings; 1982 February 15-17; Spokane, WA. Pullman, WA: Washington State University: 75-85.
Harvey, Alan E.; Jurgensen, Martin F.; Graham, Russell T. 1989. Fire-soil interactions governing site productivity in the northern Rocky Mountains. In: Baumgartner, David M.; Bruer, David W.; Zamora, Benjamin A., eds. Prescribed fire in the Intermountain Region: forest site preparation and range improvement: symposium proceedings; 1986 March 3-6; Spokane, WA. Pullman, WA: Cooperative Extension Service, Washington State University: 9-18.
Harvey, Alan E.; Jurgensen, Martin F.; Larsen, Michael J.; Graham, Russell T. 1987. Decaying organic materials and soil quality in the Inland Northwest: a management opportunity. Gen. Tech. Rep. INT-225. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 15 p.
Heilman, P. E.; Gessel, S. P. 1963. Nitrogen requirements and the biological cycling of nitrogen in Douglas-fir stands in relation to effects of nitrogen fertilization. Plant and Soil. 18: 386-402.
Hosking, J. S. 1938. The ignition at low temperatures of the organic matter in soils. Journal of Agricultural Science. 28: 393-400.
Jurgensen, M. F.; Arno, S. F.; Harvey, A. E.; Larsen, M. J.; Pfister, R. D. 1979. Symbiotic and nonsymbiotic nitrogen fixation in northern Rocky Mountain forest ecosystems. In: Gordon, J. C.; Wheeler, C. T.; Perry, D. A., eds. Symbiotic nitrogen fixation in the management of temperate forests: NSF workshop proceedings; 1979 April 2-5; Corvallis, OR: Oregon State University: 294-308.
Klopatek, C. C.; DeBano, L. F.; Klopatek, J. M. 1988. Effects of simulated fire on vesicular-arbuscular mycorrhizae in pinyon-juniper woodland soil. Plant and Soil. 109: 245-249.
Maars, R. H.; Roberts, R. D.; Skeffington, R. A.; Bradshaw, A. D. 1983. Nitrogen in the development of ecosystems. In: Lee, J. A.; McNeill, S.; Rorison, I. H., eds. Nitrogen as an ecological factor. Oxford, England: Blackwell Science Publishing: 131-137.
McGill, W. B.; Cole, C. V. 1981. Comparative aspects of cycling of organic C, N, S, and P through soil organic matter. Geoderma. 26: 267-286.
Raison, R. J.; Khanna, P. K.; Woods, P. V. 1985. Mechanisms of element transfer to the atmosphere during vegetation fires. Canadian Journal of Forest Research. 15: 132-140.
St. John, Theodore V.; Rundel, Philip W. 1976. The role of fire as a mineralizing agent in a Sierran coniferous forest. Oecologia. 25: 35-45.
Tiedemann, A. R. 1987. Combustion losses of sulfur and forest foliage and litter. Forest Science. 33: 216-223.
Trappe, J. M.; Bollen, W. B. 1979. Forest soil biology. In: Heilman, P. E.; Anderson, H. A.; Baumgartner, D. M., compilers. Forest soils of the Douglas-fir Region. Pullman, WA: Washington State University, Cooperative Extension Service: 145-151.
Turner, J. 1977. Effect of nitrogen availability on nitrogen cycling in a Douglas-fir stand. Forest Science. 23: 307-316.
Vlamis, J.; Biswell, H. H.; Shultz, A. M. 1955. Effects of prescribed burning on soil fertility in second growth ponderosa pine. Journal of Forestry. 53: 905-909.
Wells, Wade G., II. 1981. Some effects of brushfires on erosion processes in coastal southern California. In: Erosion and sediment transport in Pacific Rim steep- lands. 1981 January; Christ Church, New Zealand. Sponsored jointly by the Royal Society of New Zealand, New Zealand Hydrological Society, IAHS, and the National Water and Soil Conservation Authority of New Zealand. International Association of Hydrologic Publication Sciences. 132: 305-342.
Paper presented at the Symposium on Management and Productivity of Western-Montane Forest Soils, Boise, ID, April 10-12, 1990.
Leonard F. DeBano is Supervisory Soil Scientist, Rocky Mountain Forest and Range Experiment Station, located at the Forestry Sciences Laboratory, Arizona State University Campus, Tempe, AZ 85287; Station headquarters is in Fort Collins, CO, in cooperation with Colorado State University.