- Information by Map Unit
- Information by Topic
- Documents in SoLo
Links to other related sites
- About SoLo
-
- Mission
- Disclaimer
- Authors (complete list)
Understanding decomposition processes and the influence of forest management practices on them is crucial to maintaining long-term productivity in western forests. This paper discusses: (1) organic matter accumulations in western forests, including coarse woody debris (CWD), (2) organic matter decomposition rates, including the effects of clearcutting, (3) physical, chemical, and biological factors influencing decomposition rates, and (4) nitrogen dynamics in decomposing substrates. Decomposition rates are much higher in coastal forests (k = 0.27-0.44 yr−1 for Douglas-fir needles) than in inland forests (k = 0.05-0.14 yr−1 for pine needles). Decomposition rates for woody substrates are one to two orders of magnitude slower depending on their size. Needle decomposition rates are increased by clearcutting. Nitrogen release from a substrate is related to its decomposition rate and N may be immobilized for a long time in CWD.
Decomposition is the process whereby litter on the soil surface and belowground roots are broken down to smaller particles (Swift and others 1979). It releases soluble forms of nutrients that are available for plant uptake and provides soil organic matter (Waring and Schlesinger 1985). Understanding decomposition processes and the influence of forest management practices on them is crucial to maintaining the long-term productivity of western forests. Organic matter decomposition also contributes CO2 to the atmosphere, thus influencing global warming.
Fungi and bacteria are the dominant decomposers in coniferous forests (Richards 1987). Small animals, such as mites, fragment fine litter and enhance microbial decomposition. Earthworms, although important in the decomposition process in deciduous forests, are not thought to play a major role in coniferous forests, but they are present (Richards 1987). Insects, especially termites, ants, bark beetles, and wood borers, play a very important role in the decomposition of woody litter by fragmenting wood and introducing fungal decomposers (Harmon and others 1986).
Simple sugars decompose completely to CO2 and water, but decomposition of the complicated organic substrates in forest ecosystems is not complete. Hard-to-decompose or recalcitrant substances accumulate in the soil as humus, which comprises the soil organic matter so important in maintaining forest productivity. Soil organic matter maintains soil structure, improves soil water balance, and is a long-term source of site nutrients. It is particularly vulnerable to loss through improper forest practices and is important in preventing compaction and erosion.
There are many sources of organic matter in western forests, and one of these is coarse woody debris (CWD), such as logs and snags. Determining the importance of CWD in western forests has been the focus of much research in recent years (Harmon and others 1986; Harvey and others 1979, 1981; Larsen and others 1980; Maser and others 1988), and maintaining CWD in western forests is one of the major components of "new" forestry (Eubanks 1989; Franklin 1989). Coarse woody debris provides: (1) plant habitat through nurse logs, (2) moisture and nutrients for fine roots and mycorrhizae, (3) habitat for animals and birds, (4) pools in streams for fish habitat, (5) sites for nitrogen (N) fixation, and (6) a long-term source of soil organic matter. It also protects against erosion by improving slope and stream stability, maintains species diversity, and helps in maintaining long-term site productivity. Many of these roles change as CWD decomposes.
The objectives of this paper are to: (1) present data on organic matter accumulation in western forests, (2) discuss organic matter decomposition rates in western forests including the effects of clearcutting on decomposition rates, (3) discuss the factors influencing organic matter decomposition rates, and (4) examine N dynamics in decomposing substrates.
The sources of soil organic matter in western forests are fine litterfall (needles, leaves, insect frass, etc.), fine woody litterfall (twigs, branches, cones), coarse woody debris (logs, snags, and stumps), roots (fine and coarse), and soil organisms. The importance of fine roots in contributing organic matter to the soil has only recently been demonstrated. Fine root turnover can be equal to needle litter inputs and in some ecosystems, for example, Pacific silver fir, fine root inputs can be three times aboveground fine litter inputs (Vogt and others 1986).
The amount of dead organic matter accumulating in an ecosystem is the balance among litter and root inputs, decomposition, and the effects of fire. Organic matter accumulations in western U.S. forests are among the highest in the World's forests (Cole and Rapp 1981). There is considerable variability, however, in organic matter accumulations in this zone, with highest accumulations in productive wetter and cooler coastal areas and lowest accumulations in less productive hotter and drier inland areas (table 1). A large proportion of this accumulation is in CWD and soil organic matter. Highest CWD accumulations occur in old-growth forests in the western Olympic Mountains (> 500 Mg/ha) (Agee and Huff 1987) with lesser amounts in old-growth forests in the western Oregon and Washington Cascades (averaging 60-220 Mg/ha) (Spies and others 1988).
Fire is the major disturbance in western forests and 600-1,000 Mg/ha of CWD can be found immediately after a catastrophic wildfire in old-growth forests (Spies and others 1988). This CWD will decompose with time, and new accumulations will begin at about age 50 years. Lowest amounts of CWD (< 100 Mg/ha) tend to occur 100 to 200 years after the fire, after which the amount of CWD will increase again. Windstorms can also add large amounts of coarse woody debris in areas close to the coast.
Forest management has changed organic matter accumulations, particularly with respect to CWD (Harmon and others 1990; Spies and others 1988). Spies and others (1988) suggest forest management activities greatly reduce the amount of CWD below minimums typically encountered under natural ecosystem dynamics. For most of the managed rotation CWD biomass is < 30 Mg/ha. This is supported by data from second rotation forests in the Puget Sound area where surface CWD biomass is only around 30 Mg/ha (R. L. Edmonds, unpublished data). In third rotation forests CWD biomass is further reduced to 10 Mg/ha. Stumps left after harvest only account for about 3 Mg/ha in third-rotation forests, but coarse woody roots contribute significantly more (around 30 Mg/ha) (R. L. Edmonds, unpublished data). This loss of a long-term source of organic matter is of considerable concern.
| Location | Coastal (Douglas-fir/hemlock) |
Inland (pine) |
|---|---|---|
| 1Johnson and others (1982). 2Grier and Logan (1977). 3Fahey (1983). 4Fahey and others (1985). 5Vogt and others (1986). 6Harmon and others (1986). 7Spies and others (1988). 8Agee and Huff (1987). 9Pearson and others (1984). |
||
| Litter and humus | 1,216–57 | 3,45–33 |
| Fine roots | 55–13 | |
| Coarse woody debris | ||
| logs | 2,6,7,842–500 | 6,41–104 |
| snags | 625–105 | 62–41 |
| coarse roots | 297–193(live) | 926–56(live) |
| Fine roots | 179–776 | 4490–591 |
Soil organic matter is also likely to be reduced with forest management (Harmon and others 1990), although few studies have been conducted to examine this. The optimum level of organic matter in soils to maintain productivity in western forests is not known. However, organic matter removal on poor Douglas-fir sites tended to have a greater effect on reducing productivity than on good sites (Bigger 1988). The forest floor, roots, and fine woody litter become increasingly important contributors to soil organic matter as the intensity of forest management increases and the contribution of large woody litter decreases.
Organic matter decomposition rates are usually determined by examining substrate dry weight or mass loss, changes in specific gravity, or carbon dioxide evolution from a substrate. Substrate mass loss with time is typically used to determine decomposition rates of fine litter (needles, leaves, twigs, cones, bark, small branches, etc.), whereas specific gravity change is usually used for determining CWD decomposition rates. Although the specific gravity of large boles can be determined relatively easily, it is more difficult to determine how long a bole has been on the ground unless the bole is a result of a known blowdown. Typically, logs that have been on the ground for a long time are aged by examining adjacent living trees for scars and aging the scars using an increment borer (Sollins and others 1987). The age of trees growing on downed logs can also be determined. Both fragmentation and respiration losses have to be taken into account for boles and snags.
Carbon dioxide evolution is not typically used for determining decomposition rates of specific substrates, because it is such a dynamic measure and it is hard to integrate over a long time period. It is also difficult to separate substrate respiration from root respiration, and forest-floor CO2 evolution is not always well related to decomposition rates (Vogt and others 1980).
Typically, decomposition rates are expressed as k values or fractional loss rates rather than just changes in mass or specific gravity with time. The unit of k is typically yr −1 or per year. A simple negative exponential curve can be used to express decomposition and the k value can be determined from the equation X/Xo = e−kt, where X o = initial dry mass or specific gravity and X = mass or specific gravity at time t (years) (Olson 1963). Typically, k values using this equation decrease with time because the rate of decomposition is more rapid early in the process and slows with time (Edmonds 1984; Yavitt and Fahey 1982). More complex models have also been developed (for example, Bunnell and Tait 1974; Means and others 1982; Melillo and others 1989). The k values for CWD commonly have a fragmentation component (kfrag) and a respiration component (kresp) or (kmin) (Harmon and others 1986).
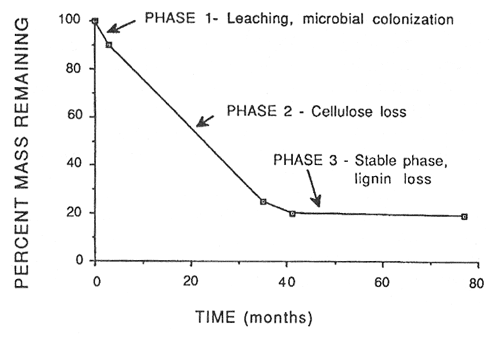
Decomposition of fine litter, such as needles or leaves, occurs in three phases as shown in figure 1 for red pine in the eastern U.S. (Melillo and others 1989). In the first phase, during the first few months, the labile or fast fraction (sugars and starch, etc.) is lost by rapid microbial assimilation or leaching. Harmon and others (1990) noted that leached litter in coastal Washington decomposed slower than unleached litter. The second phase is dominated by loss of structural or slow carbon, which is primarily cell wall polymers such as cellulose. The third phase is the stable or metastable phase in which there is a very slow decrease in mass. This phase is dominated by lignin decomposition.
Mellillo and others (1989) have examined decomposition along a decay continuum from plant litter to soil organic matter and feel that litter mass loss can best be modeled using a two-phase model: an initial phase of constant mass loss and a very slow loss dominated by a degradation of "lignocellulose" (acid-soluble sugars plus acid-insoluble C compounds). As the decaying litter enters the second phase, the ratio of lignin to lignin plus cellulose (the lignocellulose index-LCI, or the fraction of lignin in the lignocellulose complex) approaches 0.7. Beyond this the LCI increases only slowly throughout the decay continuum indicating that acid insoluble materials (lignin) dominate decay in the later stages. For red pine needles the second phase began when the organic matter remaining was about 20 percent; it ranged between 15 and 30 percent for other litter materials (Melillo and others 1989).
Figure 1—Phases of decomposition in red pine needles in Massachusetts. From Melillo and others (1989). [view larger image - 20K] [Text description of this graph]
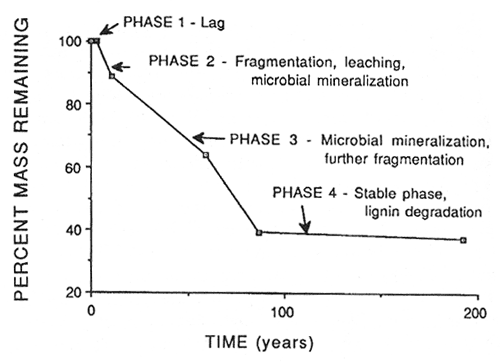
Woody litter goes through a similar process but has at least one additional stage as shown in figure 2. Before any weight loss or change in specific gravity occurs there is a lag phase (phase 1), which is usually related to the size of the substrate (larger woody substrates usually have a longer lag time). In phase 2, logs begin to weather and fragment. Leaching losses and microbial activity occur. After the period of active fragmentation there is a period of rapid microbial mineralization (phase 3). Fragmentation still proceeds during this phase. This is followed by the stable phase, which is dominated by lignin decomposition (phase 4). Most coniferous logs at this stage consist of a mass of crumbly brown cubical rot. Maser and others (1988) have described five visual decay classes for Douglas-fir logs as shown in table 2 involving the presence or absence of bark, twigs and roots, texture, shape, color, etc. Note that roots do not begin to invade logs until they reach decay class III.
Figure 2—Phases of decomposition of Douglas-fir logs in western Oregon and Washington. Data from Sollins and others (1987). [view larger image - 24K] [Text description of this figure]
The study of organic matter decomposition in western forests is of relatively recent origin. Few studies were conducted before the 1970's, and most of these involved examining bole deterioration, particularly after windstorms (Shea and Johnson 1962) or insect epidemics (Wright and Harvey 1967). They did not determine actual decomposition rates.
Interest in studying organic matter decomposition rates in western forests from an ecological viewpoint increased after the initiation of the Coniferous Forest Biome Program of the International Biological Program (IBP) in the 1970's (Edmonds 1982). A number of studies were initiated in western Oregon and Washington at that time, mostly focusing on needle decomposition (for example, Edmonds 1979, 1980, 1984; Fogel and Cromack 1977). The IBP also stimulated interest in studying decomposition rates of coarse woody debris, and a number of studies were conducted starting in the late 1970's (Graham and Cromack 1982; Grier 1978; Harmon and others 1986; Sollins 1982; Sollins and others 1987; Spies and others 1988).
Decomposition rates of needles, roots, branches, bark, and wood in western forests, including inland sites, are summarized in table 3. Douglas-fir was taken to be a representative species for coastal sites, while data for pines are presented for inland sites. There are differences, however, in decomposition rates among different species occurring in a local area. For example, in the Washington Cascades k values for low-elevation red alder, Douglas-fir, western hemlock, and high-elevation Pacific silver fir are 0.45, 0.44, 0.38, and 0.30 yr−1, respectively, based on 2 years of decomposition (Edmonds 1980).
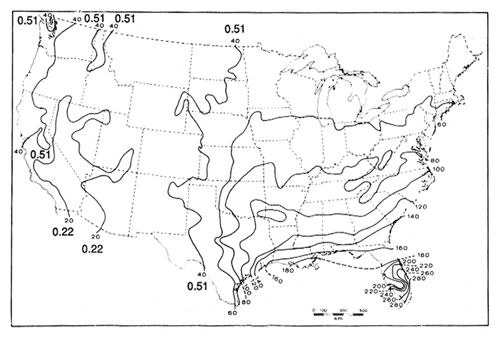
Overall decomposition rates for needles are much higher in coastal Douglas-fir (k = 0.27-0.44 yr−1) than in pine types in California (k = 0.05 yr−1), Arizona (k = 0.14 yr−1), and Wyoming (k = 0.14 yr−1), due to moister conditions in coastal forests. This is also illustrated in figure 3, which shows isolines of first-year needle and leaf decomposition rates in the U.S. The predicted rates are higher than actual rates because first-year rates are usually very rapid. Note that decomposition rates in the West are in general considerably slower than those in the eastern U.S., especially the southeastern U.S. This largely reflects different climatic regimes. It is considerably warmer and wetter in the southeastern U.S. than it is in the West and summers tend to be dry in the West even in coastal areas. The factors controlling decomposition rates are discussed in more detail in the following section. Decomposition rates also change with stand age, with the fastest rate occurring near the time of canopy closure (Edmonds 1979).
There are few data on fine root decomposition rates in the West, but the data that do exist indicate that rates are slightly slower or similar to those for foliage (table 3). Berg (1984) also noted this for Scots pine fine roots. Coarse woody roots in Wyoming decomposed more slowly (k = 0.027 yr−1) than fine roots (k = 0.05 yr−1). This k value for coarse roots, however, was calculated after 12-15 years of decomposition. When calculated after 80-110 years it decreased to 0.008 yr−1 (Yavitt and Fahey 1982). Coarse root wood appears to decay very slowly in the later stages of decomposition. Yavitt and Fahey (1982) noted there was still a large amount of root wood at their sites in Wyoming 100 years after tree death. Bark also decomposes slowly, and Douglas-fir bark decomposes at a similar rate (k = 0.03 yr−1) to small logs (table 3).
Considerably more decomposition data exist for surface woody litter. Wood decomposes one to two orders of magnitude slower than needles depending on size (table 3). Branches decompose at a faster rate than logs, and small logs decompose at a faster rate than large logs (table 3). Edmonds and Eglitis (1989), in an exception to this rule, however, noted that small Douglas-fir logs (average diameter 24 cm) decomposed at a slower rate than medium-sized logs (average diameter 37 cm). Small logs were not as easily attacked by wood-boring insects, which apparently spread wood-rotting fungi. This was only a 10-year study, however, during the initial period of decomposition.
| Substrate | Coastal (Douglas-fir) | Inland (pines) | ||
|---|---|---|---|---|
| k(yr−1)1 | Time to 95% decay (yr)2 |
k(yr−1) | Time to 95% decay (yr) |
|
| 1Based on single exponential model of Olson (1963). 23/k (Olson 1963). 3Edmonds (1980a). 4Stohlgren (1988a). 5Fogel and Cromack (1977). 6Klemmedson and others (1985). 7Yavitt and Fahey (1986). 8Edmonds (1987). 9Fogel and Hunt (1979). 10Yavitt and Fahey (1982). 11Edmonds and Eglitis (1989). 12Harmon and others (1986). 13Spies and others (1988) (includes both fragmentation and respiration). |
||||
| Needles | ||||
| 0.44 (WA)3 | 7 | 0.05 (CA)4 | 60 | |
| 0.27 (OR)5 | 11 | 0.14 (AZ)6 | 21 | |
| 0.14 (WY)7 | 21 | |||
| Branches | ||||
| 0.06 (WA)8 | 121 | |||
| Roots | ||||
| fine | 0.18 (OR)9 | 17 | 0.05 (WY)10 | 60 |
| coarse | 0.027 (WY)10 | 111 | ||
| Bark | ||||
| 0.03 (OR)5 | 100 | |||
| Wood (diam., cm) | ||||
| 24 (log) | 0.026 (WA)11 | 115 | ||
| 37 (log) | 0.050 (WA)11 | 60 | ||
| >65 (log) | 0.006 (WA, OR)12 | 500 | ||
| 0.029 (WA, OR)12 | 103 | |||
Figure 3—Isopleths of percent needle and leaf litter produced annually that will decompose in the first year. k values (yr−1) are shown for the
western U.S. Adapted from Meentemeyer (1978a). [view larger image - 56K] [Text description of this figure]
Large Douglas-fir logs in coastal forests may exist on the forest floor for more than 500 years based on a simple k value (table 3). Spies and others (1988), however, suggested that using kresp values to calculate the longevity of logs may be misleading. They found much higher k values (0.029 yr−1 ) when fragmentation losses were included, resulting in a much shorter longevity (closer to 100 years). They feel that this is a more realistic rate based on CWD accumulations in Cascade Mountain forests. Nevertheless, it is apparent that Douglas-fir logs exist for an extended period on the forest floor, performing important ecological roles as they decompose. A typical old-growth Douglas-fir tree may live for 500 years, but it continues to function for 100 years or more after death.
Logs of different tree species have slightly different de-composition rates. Sollins and others (1987) determined k values (0.009, 0.016, and 0.010 yr−1) for western red-cedar, western hemlock, and Douglas-fir, respectively. However, they also noted that no recognizable western redcedar or hemlock logs greater than 100 years old could be found, yet Douglas-fir logs almost 200 years old were recognizable. Thus, there seems to be a problem in using k values for determining exact log life.
There appears to be a lack of data for log decomposition rates for inland sites in the West (Harmon and others 1986), but the impression is that logs may not last as long in inland sites as they do in coastal sites. At inland sites logs are smaller, tend to be easily colonized by insects, and of course tend to burn more easily in an environment where the fire frequency is higher. Standing dead trees or snags may have high fragmentation decomposition rates at inland sites (kfrag = 0.073-0.318 yr−1) resulting from physical exposure and the action of insects and cavity-nesting birds (Harmon and others 1986). Snags in general tend to have higher decomposition rates than logs. For example, for Douglas-fir snags kfrag = 0.014-0.354 yr−1 and kmin = 0.003-0.027 yr−1. For Douglas-fir boles kfrag = 0.008 yr−1 and kmin = 0.004-0.007 yr−1. Note that k values for fragmentation are higher than k values for mineralization.
The influence of forest management on litter decomposition rates currently holds considerable interest, especially decomposition rates in clearcuts. Table 4 shows that decomposition rates of Douglas-fir in Washington and pine needles in Arizona are higher in clearcuts than in closed-canopy stands. Entry and others (1987) also found higher decomposition rates for needles in clearcuts in Montana. Decomposition rates may also be higher at forest edges than beneath closed canopy stands (table 4). This has important implications with respect to nutrient availability and tree growth. In contrast to needles, twigs and branches in clearcuts seem to have a slower rate of decomposition than those in closed canopy stands (table 4), perhaps because they dry out rapidly in clearcuts.
| Material | Closed canopy | Clearcut | Forest edge | |
|---|---|---|---|---|
| 1Edmonds (1980). 2Edmonds and Bigger (1984). 3Klemmedson and others (1985). 4Edmonds and others (1986). 5Erickson and others (1985). |
||||
| Douglas-fir needles (WA)1,2 | 0.44 | 0.39-0.53 | 0.29-0.51 | |
| Ponderosa pine needles (AZ)3 | 0.14 | 0.34 | ||
| Douglas-fir twigs and branches (WA)4,5 | 0.06-0.14 | 0.01-0.04 | ||
| Ponderosa pine (WA)5 | 0.01 | |||
Many interacting factors are involved in the decomposition of organic substrates. A list of physical, chemical, and biological factors influencing decomposition is shown in table 5; these factors are discussed below.
Temperature and moisture play important roles in the decomposition process. Decomposition is generally faster in cool, moist areas and slower in hot, dry areas, and this is generally the case in the western U.S. as illustrated by the data in table 3 and figure 1. There is tremendous variation in temperature and moisture in the West because of the mountainous terrain. Elevation and slope factors can change temperature and moisture conditions over relatively short distances. Forest management effects such as clearcutting also can dramatically change temperature and moisture conditions. Moisture conditions in clearcuts in the early summer may be more conducive for decomposition in clearcuts because of lower Evapotranspiration. Meentemeyer (1978b) found that decomposition rates were negatively related to actual Evapotranspiration. Temperatures are also warmer in clearcuts.
Substrate size or surface-to-volume ratio is also important. Fine litter has a high surface-to-volume ratio and is more easily colonized by microbes than woody substrates. Very large diameter logs decompose slowly (table 3), reflecting their small surface-to-volume ratios. Decomposition of woody substrates, however, is not strictly determined by surface-to-volume ratio. Insects are extremely important in wood decomposition, and certain groups of insects, such as wood borers, may not be as effective in attacking small logs as larger logs, thus influencing decomposition rates.
Substrate location is also very important. Buried wood, for example, decomposes much faster than wood on the surface of clearcuts with aerial wood decomposing even more slowly (Edmonds and others 1986). This largely reflects different temperature and moisture conditions. Decomposition rates are also lower in locations with low aeration and high CO2(Griffin 1972), for example, in very wet soils. Decomposition proceeds more slowly in anaerobic environments because fungi are excluded and only bacteria are present.
Fire is not usually considered as an agent of decomposition. It does, however, oxidize organic matter and could be considered a decomposing agent in hot, dry ecosystems where microbial decomposition is slow. It may also influence the ability of substrates to microbially decompose after a fire. Most fires completely oxidize fine litter, but charred woody substrates may remain. Little information is available on decomposition rates of charred logs, but observation would suggest that charred logs decompose at a slower rate than noncharred logs. Certainly charcoal can remain in the soil for centuries after a fire. Entry and others (1987) found that lignin and cellulose decomposition was slower in clearcut and burned sites in Montana than in unburned clearcuts.
Considerable research has been conducted on the influence of substrate chemistry on decomposition rates. Generally, substrates with high N concentrations and low C:N ratios decompose at the fastest rate. However, the relationship is more complex than this, and as a result attention has focused on other factors such as substrate structural chemistry. Plant substrates are made up of noncell-wall components (waxes, resins, phenolics, sugars) and cell-wall structural chemicals (cellulose and lignin). Slowly decomposing substrates are generally high in lignin (Fogel and Cromack 1977). Melillo and others (1982) suggested that both lignin and N (lignin:N ratio) may be a better predictor of decomposition rates than lignin or N alone. The lignocellulose index also appears to be related to decomposition rates (Melillo and others 1989).
The concentration of extractives (for example, phenolics) in substrates is also important in determining decomposition rates (Swift and others 1979). Extractives are materials that can be removed by solvents such as hot water, ethanol, or benzene. Red alder leaves in coastal Washington decompose slower than predicted by the lignin:N ratio (Harmon and others 1990) perhaps because of their high extractives content.
Extractives are particularly important in controlling decomposition rates of woody substrates. They typically give color to heartwood. Thus species with dark-colored heartwood, such as redwood, Douglas-fir, and western red cedar, are typically more decay resistant than white wood species such as hemlocks and true firs.
Acidity or pH can also influence decomposition rates. At low pH, microbes are inhibited, especially bacteria (Richards 1987).
Biological factors are extremely important in the decomposition process. Without the presence of microbes and their enzymes, organic matter decomposition would be very slow (Ugolini and Edmonds 1983). Fungi are the dominant agents of decomposition in aerobic environments. They possess enzymes such as cellulase to more efficiently break down complex substrates, and they can tolerate more acidic environments than bacteria. Organic acids are produced during the decomposition process, which tend to result in an acid soil environment, particularly in cold environments.
Mycorrhizae are important in western forests with respect to nutrient uptake by plants (Amaranthus and others 1989; Maser and others 1988). However, it has been suggested by workers in New Zealand that the presence of mycorrhizae may inhibit litter-decomposing fungi (Gadgil and Gadgil 1975). This, however, has not as yet been demonstrated in western U.S. forests. Griffiths and others (1990), however, have demonstrated complex interactions among Douglas-fir trees, ectomycorrhizal fungi, and the microbial community in ectomycorrhizal mats in the soil.
Soil animals such as earthworms are important in decomposition of most deciduous leaf litter (Richards 1987). Although native earthworms are present in acidic western coniferous ecosystems, they are generally not thought to play an important role in the decomposition process. Spiers and others (1986), however, argue that the role of indigenous earthworms in decomposition and nutrient cycling in Northwest coastal ecosystems may have been underestimated. They observed as many as 200 worms m2 in some ecosystems. Other dominant soil animals in coniferous forests are the enchytraeid worms, mites, and insects such as Collembola, which tend only to fragment organic matter or graze on fungi and bacteria (Edmonds 1980b).
Although insects may be not be very important in the decomposition of fine litter, they play an extremely important role in wood decomposition, particularly in the early phases (Carpenter and others 1988; Edmonds and Eglitis 1989). They fragment woody substrates and introduce fungal spores and mycelia. Carpenter ants, bark beetles, wood borers, and termites are the dominant insects involved.
Finally, in terms of biological factors, plant species is important (Daubenmire and Prusso 1963). Each species has different substrate chemistries in needles, leaves, roots, branches, and boles, and this strongly influences their decomposition rates (Edmonds 1980a; Sollins and others 1987).
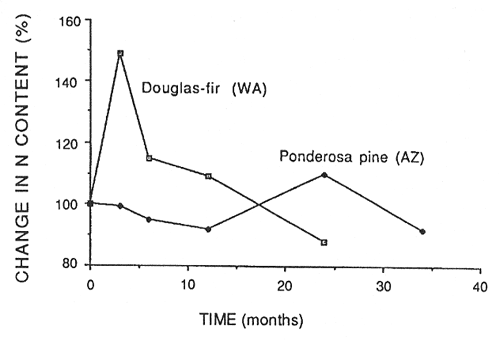
Nitrogen is the major growth-limiting nutrient element in the Northwest (Edmonds and others 1989), and its availability is largely determined by its rate of release from decomposing organic matter. Fresh litter falling to the forest floor is decomposed by fungi and bacteria, which typically immobilize any N in the decomposing substrate for a period of time before releasing it to the soil where it becomes available for plant uptake. Figure 4 illustrates the fate of N with time in decomposing Douglas-fir needles in Washington (Edmonds 1980a) and ponderosa pine needles in Arizona (Klemmedson and others 1985). Nitrogen was immobilized in Douglas-fir needles only in the first 3 months, while it appeared to be immobilized in ponderosa pine needles for 24 months. Stohlgren (1988b) also noted a long period of immobilization in needle litter in some Sierra Nevada ecosystems in California.
Figure 4—Percent of original N mass in decomposing Douglas-fir needles in Washington (Edmonds 1980a) and ponderosa pine needles in Arizona (Klemmedson and others 1985) in relation to time. [view larger image - 16K] [Text description of this figure]
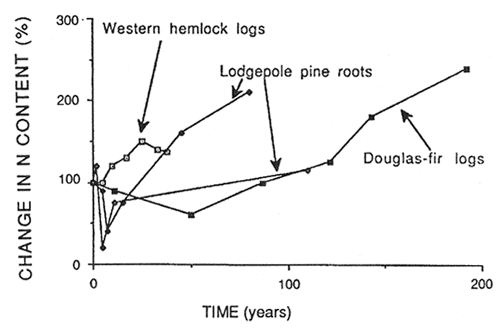
The length of this period of immobilization depends on the concentration of N in the initial substrate. Substrates high in N, such as red alder leaves, release N almost immediately on reaching the forest floor (Edmonds 1980a), while logs that have a low N concentration may immobilize N for as long as 25 years (Grier 1978) or even 190 years (Sollins and others 1987) (fig. 5). Yavitt and Fahey (1982) found that the N content of decaying woody roots was still increasing after more than 100 years of decomposition (fig. 5).
Figure 5—Percent of original N mass in decomposing western hemlock logs in coastal Oregon (Grier 1978), Douglas-fir logs in the Washington and Oregon Cascades (Sollins and others 1987) and lodgepole pine woody roots in Wyoming (Yavitt and Fahey 1982) in relation to time. [view larger image - 24K] [Text description of this figure]
The C:N ratio of decomposing substrates continuously declines with time and with depth in the soil profile. For example, it may be 50:1 in freshly fallen Douglas fir needles (Edmonds 1980a), below 30:1 in the forest floor, and less than 20:1 in the soil (Edmonds and Hsiang 1987). A critical C:N ratio has been suggested as controlling N release in decomposing substrates. Lutz and Chandler (1946) suggested that N mineralization should occur at C:N ratios between 20 and 30:1 with immobilization occurring at ratios greater than this. This appears to be the case for decomposing leaf and needle litter (Edmonds 1980, 1984), but it does not appear to be the case for woody substrates. Critical C:N ratios for N mineralization appear to be > 100:1 for twigs and branches and > 300:1 in large logs (Edmonds 1987; Sollins and others 1987). Thus the critical C:N ratio for N release is not constant but increases as the substrate decomposition rate decreases. There also appears to be no N release until lignin decomposition starts (Berg and McClaugherty 1987). The association between N release from the litter late in decay and the breakdown of the lignocellulose complex is not clearly understood, however, and deserves further study (Melillo and others 1989).
Despite the large biomass of woody debris in logs on the forest floor, the major supply of N for plants probably comes from the fine litter because its N concentration and content is so much higher than that of logs. Logs, however, should not be discounted in the N cycling story. Nitrogen fixation occurs in decomposing logs (Larsen and others 1980; Silvester and others 1982), particularly in decay classes II-IV, and it is important in the overall N balance of a site over the length of a rotation. Sollins and others (1987) noted that a symbiotic bacteria in fallen logs fix about 1 kg ha−1 yr−1 of N in the Oregon and Washington Cascades. This is a substantial amount relative to other system N inputs from precipitation and dry fall (2-3 kg N ha−1 yr−1). Nitrogen in logs is available for fine roots and mycorrhizae to take up at much higher C:N ratios (> 300:1) than that in needles (20-30:1) and this, in combination with a favorable moisture regime, may explain why plants are able to grow so easily on CWD. Nitrogen release in Douglas-fir logs appears to coincide with the onset of lignin decay (Kermit Cromack, Department of Forest Science, Oregon State University, personal communicaton).
Large organic matter accumulations occur in western forests with higher accumulations in coastal than inland forests; higher productivity ecosystems have higher accumulations, particularly of CWD. Accumulations represent a balance among inputs, decomposition, and the effects of fire. Forest management has reduced the amount of coarse woody debris below that encountered in natural ecosystems, and this is of some concern with respect to the maintenance of long-term productivity in managed ecosystems.
Decomposition rates are much slower in inland than in coastal forests. Woody litter decomposes one to two orders of magnitude slower than needles. Despite its slow decomposition rate, however, woody litter provides a long-term source of soil organic matter and nitrogen, which could be important in maintaining site productivity. The decomposition process and its relation to forest productivity, however, is still incompletely understood.
Decomposition rates of litter are primarily determined by physical factors (especially temperature, moisture, and surface-to-volume ratios), substrate chemistry (especially lignin concentration), and biological factors (especially the presence of microbes and insects and plant species). Insects are particularly important in wood decomposition.
Clearcutting appears to increase the rate of fine litter decomposition, but may slow woody litter decomposition. Fine litter decomposition also appears more rapid in forest edges than in closed-canopy stands.
Nitrogen in fine surface litter is released relatively rapidly compared to release in CWD, where it is immobilized for many years. The rate of N release appears to be related to the rate of decomposition. Nitrogen does, however, become available to plants in large woody substrates at much higher C:N ratios (> 300:1) than in needle litter.
Agee, J. K.; Huff, M. H. 1987. Fuel succession in a western hemlock/Douglas-fir forest. Canadian Journal of Forest Research. 17: 697-704.
Amaranthus, M. P.; Trappe, J. M.; Molina, R. J. 1989. Long-term productivity and the living soil. In: Perry, D. A.; Meurisse, R.; Thomas, B.; Miller, R.; Boyle, J.; Means, J.; Perry, C. R.; Powers, R. F., eds. Maintaining the long-term productivity of Pacific Northwest forest ecosystems. Portland, OR: Timber Press: 36-52.
Berg, B. 1984. Decomposition of root litter and some factors regulating the process: long-term root litter decomposition in a Scots pine forest. Soil Biology and Biochemistry. 16: 609-617.
Berg, B.; McClaughtery, C. 1987. Nitrogen release from litter in relation to disappearance of lignin. Biogeochemistry. 4: 219-224.
Bigger, C. M. 1988. Effects of harvest intensity on nutrient removal, nutrient leaching and growth of seedlings in individual stands of high and low productivity red alder and Douglas-fir. Seattle, WA: University of Washington. 178 p. Dissertation.
Bunnell, F. L.; Tait, D. E. N. 1974. Mathematical models of the decomposition process. In: Holding, J. A.; Heal, O. W.; MacLean, S. F., Jr.; Flanagan, P. W. Soil organisms and decomposition in tundra. Stockholm, Sweden: Tundra Biome Steering Committee: 207-225.
Carpenter, S. E.; Harmon, M. E.; Ingham, E. R.; Kelsey, R. G.; Lattin, J. D.; Schowalter, T. D. 1988. Early patterns of heterotroph activity in conifer logs. Proceedings of the Royal Society of Edinburgh. 94B: 33-43.
Cole, D. W.; Rapp, M. 1981. Elemental cycling in forest ecosystems. In: Reichle, D. E., ed. Dynamic properties of forest ecosystems. IBP 23. Cambridge, England: Cambridge University Press: 341-409.
Daubenmire, R.; Prusso, D. C. 1963. Studies of the decomposition rates of tree litter. Ecology. 44: 589-592.
Edmonds, R. L. 1979. Decomposition and nutrient release in Douglas-fir needle litter in relation to stand development. Canadian Journal of Forest Research. 9: 132-140.
Edmonds, R. L. 1980a. Litter decomposition and nutrient release in Douglas-fir, red alder, western hemlock and Pacific silver fir ecosystems in western Washington. Canadian Journal of Forest Research. 10: 327-337.
Edmonds, R. L. 1980b. Microbial life in the soil. Biology Digest. 7(2): 11-20.
Edmonds, R. L., ed. 1982. Analysis of coniferous forest ecosystems in the western United States. Stroudsburg, PA: Hutchinson Ross Publishing Company. 419 p.
Edmonds, R. L. 1984. Long-term decomposition and nutrient dynamics in Pacific silver fir needles in western Washington. Canadian Journal of Forest Research. 14: 395-400.
Edmonds, R. L. 1987. Decomposition rates and nutrient dynamics in small-diameter woody litter in four forest ecosystems in Washington, U.S.A. Canadian Journal of Forest Research. 17: 499-509.
Edmonds, R. L.; Bigger, C. M. 1984. Decomposition and nitrogen mineralization rates in Douglas-fir needles in relation to whole tree harvesting practices. In: Proceedings 1983 Society of American Foresters Annual Convention, Portland, OR. Washington, DC: Society of American Foresters: 187-192.
Edmonds, R. L.; Eglitis, A. 1989. The role of the Douglas-fir beetle and wood borers in the decomposition of and nutrient release from Douglas-fir logs. Canadian Journal of Forest Research. 19: 853-859.
Edmonds, R. L.; Hsiang, T. 1987. Influence of forest floor and soil properties on response of Douglas-fir to urea. Soil Science Society of America Journal. 57: 1332-1337.
Edmonds, R. L.; Vogt, D. J.; Sandberg, D. H.; Driver, C. H. 1986. Decomposition of Douglas-fir and red alder wood in clear-cuttings. Canadian Journal of Forest Research. 16: 822-831.
Edmonds, R. L.; Binkley, D.; Feller, M. C.; Sollins, P.; Abee, A.; Myrold, D. D. 1989. Nutrient cycling: effects on productivity of Northwest forests. In: Perry, D. A.; Meurisse, R.; Thomas, B.; Miller, R.; Boyle, J.; Means, J.; Perry, C. R.; Powers, R. F., eds. Maintaining the long-term productivity of Pacific Northwest forest ecosystems. Portland, OR: Timber Press: 17-35.
Entry, J. A.; Stark, N. M.; Loewenstein, H. 1987. Timber harvesting: effects on degradation of cellulose and lignin. Forest Ecology and Management. 22: 79-88.
Erickson, H. E.; Edmonds, R. L.; Petersen, C. E. 1985. Decomposition of logging residues in Douglas-fir, western hemlock, Pacific silver fir and ponderosa pine ecosystems. Canadian Journal of Forest Research. 15: 914-921.
Eubanks, S. 1989. Applied concepts of ecosystem management: developing guidelines for coarse woody debris. In: Perry, D. A.; Meurisse, R.; Thomas, B.; Miller, R.; Boyle, J.;Means, J.; Perry, C. R.; Powers, R. F., eds. Maintaining the long-term productivity of Pacific Northwest forest ecosystems. Portland, OR: Timber Press: 230-236.
Fahey, T. J. 1983. Nutrient dynamics of aboveground detritus in lodgepole pine (Pinus contorta ssp. latifolia) ecosystems, southeastern Wyoming. Ecological Monographs. 53: 51-72.
Fahey, T. J.; Yavitt, J. B.; Pearson, J. A.; Knight, D. H. 1985. The nitrogen cycle in lodgepole pine forests, southeastern Wyoming. Biogeochemistry. 1: 257-275.
Fogel, R.; Cromack, K., Jr. 1977. Effect of habitat and substrate quality on Douglas-fir litter decomposition in western Oregon. Canadian Journal of Botany. 55: 1632-1640.
Fogel, R.; Hunt, G. 1979. Fungal and arboreal biomass in a western Oregon Douglas-fir ecosystem: distribution patterns and turnover. Canadian Journal of Forest Research. 9: 245-256.
Franklin, J. F. 1989. Toward a new forestry. American Forests. 95(11 and 12): 37-44.
Gadgil, R. L.; Gadgil, P. D. 1975. Suppression of litter decomposition by mycorrhizal roots of Pinus radiata. New Zealand Journal of Forest Science. 5: 33-41.
Graham, R. L.; Cromack, K., Jr. 1982. Mass, nutrient content and decay rate of dead boles in rain forests of Olympic National Park. Canadian Journal of Forest Research. 12: 511-521.
Grier, C. C. 1978. A Tsuga heterophylla-Picea sitchensis ecosystem of coastal Oregon: decomposition and nutrient balances of fallen logs. Canadian Journal of Forest Research. 8: 198-206.
Grier, C. C.; Logan, R. S. 1977. Old-growth Pseudotsuga menziesii communities of a western Oregon watershed: biomass distribution and production budgets. Ecological Monographs. 47: 373-400.
Griffin, D. M. 1972. Ecology of soil fungi. Syracuse, NY: Syracuse University Press. 193 p.
Griffiths, R. P.; Caldwell, B. A.; Cromack, K., Jr.; Morita, R. Y. 1990. Douglas-fir forest soils colonized by ectomycorrhizal mats. I. Seasonal variation in nitrogen chemistry and nitrogen cycle transformation rates. Canadian Journal of Forest Research. 20: 211-218.
Harmon, M. E.; Anderson, N. H.; Franklin, J. F.; Cline, S. P.; Swanson, F. J.; Aumen, N. G.; Sollins, P.; Sedell, J. R.; Gregory, S. V.; Lienkaemper, G. W.; Lattin, J. D.; Cromack, K., Jr.; Cummins, K. W. 1986. Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research. 15: 133-302.
Harmon, M. E.; Baker, G. A.; Spycher, G.; Greene, S. A. 1990. Leaf-litter decomposition in Picea/Tsuga forests of Olympic National Park, Washington, U.S.A. Forest Ecology and Management. 31: 55-66.
Harmon, M. E.; Ferrell, W. K.; Franklin, J. F. 1990. Effects on carbon storage of conversion of old-growth forests to young forests. Science. 247: 699-702.
Harvey, A. E.; Jurgensen, M. F.; Larsen, M. J. 1979. Role of forest fuels in the biology and management of soil. Gen. Tech. Rep. INT-65. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 8 p.
Harvey, A. E.; Jurgensen, M. F.; Larsen, M. J. 1981. Organic reserves: importance to ectomycorrhizae in forest soils of western Montana. Forest Science. 27: 442-445.
Johnson, D. W.; Cole, D. W.; Bledsoe, C. S.; Cromack, K.; Edmonds, R. L.; Gessel, S. P.; Grier, C. C.; Richards, B. N.; Vogt, K. A. 1982. Nutrient cycling in forests of the Pacific Northwest. In: Edmonds, R. L., ed. Analysis of coniferous forest ecosystems in the western United States. Stroudsburg, PA: Hutchinson Ross Publishing: 186-232.
Klemmedson, J. O.; Meier, C. E.; Campbell, R. E. 1985. Needle decomposition and nutrient release in ponderosa pine ecosystems. Forest Science. 31: 647-660.
Larsen, M. J.; Harvey, A. E.; Jurgensen, M. F. 1980. Residue decay processes and associated environmental functions in Northern Rocky Mountain forests. In: Proceedings of a symposium on environmental consequences of timber harvesting in Rocky Mountain coniferous forests. Gen. Tech. Rep. INT-90. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 157-174.
Lutz, H. J.; Chandler, R. F., Jr. 1946. Forest soils. New York: John Wiley and Sons. 514 p.
Maser, C.; Tarrant, R. F.; Trappe, J. M.; Franklin, J. F., tech eds. 1988. From the forest to the sea: a story of fallen tress. Gen. Tech. Rep. PNW-229. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 153 p.
Means, J. E.; Cromack, K., Jr.; MacMillan, P. C. 1986. Comparison of decomposition models using wood density of Douglas-fir logs. Canadian Journal of Forest Research. 15: 1092-1098.
Meentemeyer, V. 1978a. Climatic regulation of decomposition rates of organic matter in terrestrial ecosystems. In: Adriano, D. C.; Brisbin, I. Lehr, Jr., eds. Environmental chemistry and cycling processes: Proceedings of a symposium, 1976 April 28-May 1. Washington, DC: Technical Information Center, U.S. Department of Energy, CONF-760429: 779-789.
Meentemeyer, V. 1978b. Macroclimate and lignin control of litter decomposition rates. Ecology. 59: 465-472.
Melillo, J. M.; Aber, J. D.; Linkins, A. E. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology. 63: 621-626.
Melillo, J. M.; Aber, J. D.; Linkins, A. E.; Ricca, A.; Fry, B.; Nadelhoffer, K. J. 1989. Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant and Soil. 115: 189-198.
Olson, J. S. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 44: 322-331.
Pearson, J. A.; Fahey, T. J.; Knight, D. H. 1984. Biomass and leaf area in contrasting lodgepole pine forests. Canadian Journal of Forest Research. 14: 259-265.
Richards, B. N. 1987. The microbiology of terrestrial ecosystems. Longman, England: Longman Scientific and Technical, Harlow, Essex, England. New York: John Wiley and Sons. 399 p.
Shea, K. R.; Johnson, N. E. 1962. Deterioration of wind-thrown conifers three years after blowdown in Southwestern Washington. For. Resour. Notes No. 44. Centralia, WA: Weyerhaeuser Timber Company. 17 p.
Silvester, W. B.; Sollins, P.; Verhoeven, T.; Cline, S. 1982. Nitrogen fixation and acetylene reduction in decaying conifer boles: effects of incubation time, aeration, and moisture content. Canadian Journal of Forest Research. 12: 646-652.
Sollins, P. 1982. Input and decay of coarse woody debris on coniferous stands in western Oregon and Washington. Canadian Journal of Forest Research. 12: 18-28.
Sollins, P.; Cline, S. P.; Verhoeven, T.; Sachs, D.; Spycher, G. 1987. Patterns of log decay in old-growth Douglas-fir forests. Canadian Journal of Forest Research. 17: 1585-1595.
Spiers, G. A.; Gagnon, D.; Nason, G. E.; Packee, E. C.; Lousier, J. D. 1986. Effects and importance of indigenous earthworms on decomposition and nutrient cycling in coastal forest ecosystems. Canadian Journal of Forest Research. 16: 983-989.
Spies, T. A.; Franklin, J. F.; Thomas, T. B. 1988. Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology. 69: 1689-1702.
Stohlgren, T. J. 1988a. Litter dynamics in two Sierran mixed conifer forests. I. Litterfall and decomposition rates. Canadian Journal of Forest Research. 18: 1127-1135.
Stohlgren, T. J. 1988b. Litter dynamics in two Sierran mixed conifer forests. II. Nutrient release from decomposing litter. Canadian Journal of Forest Research. 18: 1136-1144.
Swift, M. J.; Heal, O. W.; Anderson, J. M. 1979. Decomposition processes in terrestrial ecosystems. Berkeley, CA: University of California Press. 372 p.
Ugolini, F. C.; Edmonds, R. L. 1983. Soil biology. In: Wilding, L. P.; Smeck, N. E.; Hall, G. F., eds. Pedogenesis and soil systematics. I. Concepts and interactions. Amsterdam: Elsevier: 193-231.
Vogt, K. A.; Grier, C. C.; Vogt, D. J. 1986. Production, turnover, and nutrient dynamics of above- and belowground detritus of world forests. Advances in Ecological Research. 15: 303-377.
Vogt, K. A.; Edmonds, R. L.; Antos, G.; Vogt, D. J. 1980. Comparisons between carbon dioxide evolution, ATP concentrations and decomposition in red alder, Douglas-fir, western hemlock and Pacific silver fir ecosystems in western Washington. Oikos. 35: 72-79.
Waring, R. H.; Schlesinger, W. H. 1985. Forest ecosystems concepts and management. New York: Academic Press. 340 p.
Wright, K. H.; Harvey, G. M. 1967. The deterioration of beetle-killed Douglas-fir in western Oregon and Washington. Res. Pap. PNW-50. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 20 p.
Yavitt, J. B.; Fahey, T. J. 1986. Long-term litter decay and forest floor leaching in Pinus contorta ecosystems, southeastern Wyoming. Journal of Ecology. 74: 525-545.
Yavitt, J. B.; Fahey, T. J. 1982. Loss of mass and nutrient changes of decaying woody roots in lodgepole pine forests, southeastern Wyoming. Canadian Journal of Forest Research. 12: 745-752.
Speakers answered questions from the audience after their presentations. Following are the questions and answers on this topic:
Q. (from Dave Gillman) If the rates of decomposition are so slow, why isn't the organic accumulation on the soil surface thicker and why isn't there more woody debris in undisturbed areas?
A. The amount of dead organic matter accumulating in a system is a balance among fine litter and coarse woody debris inputs, the rate of decomposition, and the effects of fire. In ecosystems with very slow decomposition because of moist, cold conditions, for example, at high elevations in the Washington Cascades, organic inputs to the soil are high and accumulatons on the soil suface are large, including large amounts of woody debris. Fire frequency is also low in these forests. In ecosystems that have a slow decomposition rate because they are hot and dry, organic inputs to the soil are low, resulting in low surface organic accumulations despite the low decomposition rate. Furthermore, frequent fire in these ecosystems prevents large organic accumulations.
Paper presented at the Symposium on Management and Productivity of Western-Montane Forest Soils, Boise, ID, April 10-12, 1990.
Robert L. Edmonds is Professor of Soil Microbiology and Forest Pathology, College of Forest Resources, University of Washington, Seattle, WA 98195