- Information by Map Unit
- Information by Topic
- Documents in SoLo
Links to other related sites
- About SoLo
-
- Mission
- Disclaimer
- Authors (complete list)
The distribution of ectomycorrhizal activities in forest soils and their potential interactions with conifer growth in a variety of ecological/age/treatment situations are described. In old-growth ecosystems ectomycorrhizal roots are concentrated in organic soil horizons. In planted situations positive correlations between early seedling growth and ectomycorrhizal activity were consistent the first year and most often associated with high organic matter soils and competition thereafter. Potential effects of a variety of management actions on soil-related ectomycorrhizal inputs to productivity, and the opportunities for ectomycorrhizal technology to improve early performance in regenerated forest stands are discussed.
Widespread recognition of the critical role of symbiont activities, particularly ectomycorrhizae, in survival, growth, and long-term productivity of vegetative components in wildland ecosystems has provided great impetus for research on the effects of management actions on these microbial activities (Amaranthus and others 1989; Harvey and others 1979a, 1987; Jurgensen and others 1979; Perry and others 1987, 1989). Soil conditions are particularly important in governing the ability of ectomycorrhizal fungi and their respective hosts to initiate the association (Amaranthus and others 1989; Bjorkman 1970; Page-Dumroese and others 1990; Perry and others 1987; Slankis 1974) and, as we shall see, are also likely to directly affect the functioning of the association. Thus, forestry operations that substantially alter soil conditions, particularly surface horizons, are likely to alter both initiation and function of ectomycorrhizal associations and, subsequently, growth, competitive ability (St. John and Coleman 1983), and succession in the regenerated forest.
Extensive examinations of the distribution of forest tree feeder roots (Grier and others 1981; McKay and Malcolm 1988; Vogt and others 1981, 1982, 1983) and ectomycorrhizae in forested ecosystems (Fogel and Hunt 1983; Harvey and others 1976b, 1979b, 1987; St. John and Coleman 1983; Vogt and others 1982) have shown they tend to occur in shallow, relatively fertile organic horizons where they are highly subject to disturbance (fig. 1). Frequently, decayed woody deposits in and on the soil are important to ectomycorrhizal associations, particularly during dry seasons and on dry sites (Harvey and others 1978, 1979b). Decaying logs and soil wood have been recognized as unique components of ecosystems with important ties to ectomycorrhizal activity and potentially to productivity in both inland (Harvey and others 1976b, 1979a, 1987, 1989; Larsen and others 1980) and Pacific forests (Harmon and others 1986; Maser and others 1985, 1988).
Figure 1—Distribution (percentage) of ectomycorrhizae among soil horizons in western Montana (from Harvey and others 1976b). [view larger image - 64K]
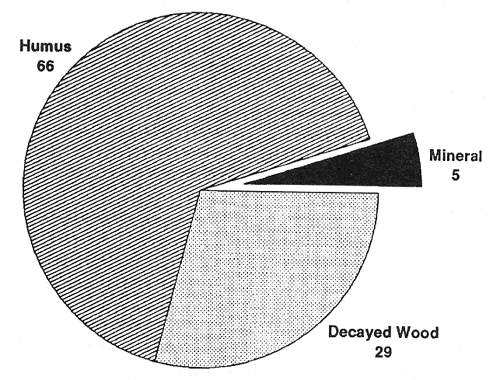
Potential for interruption in the development or destruction of surface organic horizons and resulting effects on ectomycorrhizal activities lead to a concern that inappropriate use of prescribed fire and intensive utilization on the relatively infertile soils and harsh forests of the inland West could pose a substantial danger to future productivity (Harvey and others 1976a, 1979a; Jurgensen and others 1979). Similarly, there has been concern that site preparation could also disrupt surface organic horizons and related productivity and regeneration potential of inland western forest soils (Graham and others 1989b; Harvey 1982).
The strong differential relationship between soil organic and mineral horizons and ectomycorrhizal activities under differing conditions and on differing sites (Alvarez and others 1979; Harvey and others 1978, 1979b) suggested the possibility that function of the ectomycorrhizal association, as well as initiation of the relationship, might be controlled by soil microsite conditions. The cost in energy to trees for maintaining this association, both at the stand (Fogel and Hunt 1983; Perry and others 1987) and individual tree/seedling level (Marshall and Perry 1987; Miller and others 1989), can be very high. However, tree growth may or may not be directly improved by the presence of ectomycorrhizal roots. Although in many instances presence of ectomycorrhizal activity determines both survival and growth potential for conifers (Amaranthus and Perry 1987; Perry and others 1987; Trappe and Strand 1969), in other instances there has not been a demonstrably positive effect (Krop and others 1985; Molina 1982; Shaw and others 1987; Sidle and Shaw 1987). Thus, specific information is not available regarding exactly when and where ectomycorrhizal relationships are most critical to tree survival and growth and when and where they may or may not have an immediate, critical role in the development of forest stands.
As a result of this lack of or conflicting information, we developed an experiment where site preparation treatments were applied to native inland western forest soils to provide an analysis of the effects of soil microsite conditions on both numbers of ectomycorrhizae and their impact on tree growth. Some early results of this experiment are presented here.
The objectives for the experiment were to: (1) create a situation where seedlings of two important conifer species with widely differing strategies for site adaptation (sharp vs. gradual genetic clines) could be grown in disturbed, native, forest soils while being subjected to different levels of stress as a consequence of both environment (site preparation treatment) and competing vegetation, and (2) assess ectomycorrhizal development on the planted seedlings and relate that development to performance. Since organic matter, moisture, and nutrition were known to affect ectomycorrhizal development, and stress was likely to impact ectomycorrhizal function, they were incorporated into the basic experimental design.
The experiment was conducted on two different sites located in the Priest River Experimental Forest in northern Idaho. One was located on a low bench (715 m) with relatively poor soil (Typic Xerochrept), subject to extremes of temperature and moisture, with well-developed grass, sedge, and herb competition (harsh site). This site was typical of the grand fir/snowberry (Abies grandis/Symphoricarpos albus) habitat type in northern Idaho. The other was located on a midslope (1,456 m) not subjected to such extremes of temperature and moisture, with a relatively good soil (Typic Cryothent), and lacking the well-developed competitive community (moderate site). This site was typical of the western hemlock/clintonia (Tsuga heterophylla/Clintonia uniflora) habitat type in northern Idaho. See Cooper and others (1987) for more details on these habitat types.
Superimposed onto these sites were four site preparation treatments: (1) mounded soil beds where soil organic matter volume was increased and competing vegetation left in place; (2) mounded soil beds with competing vegetation removed, manually in year 1, with herbicide (isopropylamine salt of glyphosate, 1.68 kg/ha) applied annually to non conifer vegetation only in years 2 and 3; (3) a scalp where competing vegetation, organic horizons, and mineral topsoil were removed to a depth of 5 cm; and (4) a minimally scarified area that was undisturbed after harvesting. Two randomized complete block experiments were established on each of the sites; at the low-elevation site there were four replications and on the midslope site three replications of each treatment.
Each site/treatment combination was planted with 240 1-year-old, containerized Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) or western white pine (Pinus monticola Dougl. ex D. Don) seedlings in April 1983. All seedlings were grown from locally collected seed and were essentially devoid of ectomycorrhizae at the time of planting. Sixteen randomly selected, live seedlings were subsequently excavated from each treatment at approximately 6-week intervals, starting in May and continuing through the growing season. Four sampling times were included: (1) spring, (2) early summer, (3) late summer, and (4) fall for each of the first 3 years after planting. These sampling times were equivalent to early May, mid-June, early August, and mid-September, respectively. All ectomycorrhizal root tips were counted by type (Harvey and others 1976b, 1979b); root and top dry weights (24 h at 105 C) were recorded for each seedling. Soil conditions around each of the seedlings were fully characterized, including physical and chemical parameters (Page-Dumroese and others 1986, 1989). Survival rates by treatment were also recorded (Graham and others 1989a).
Analysis of variance (Steele and Torrie 1960) was used to test the effects of site, tree species, site preparation, soil, and sample time (season/year) on numbers of ectomycorrhizae. Scheffe's multiple range test was used to detect significant differences among means (Mize and Schultz 1985). Pearson's correlation (SASS) was used to determine relationships between numbers of ectomycorrhizal short roots and seedling growth characteristics.
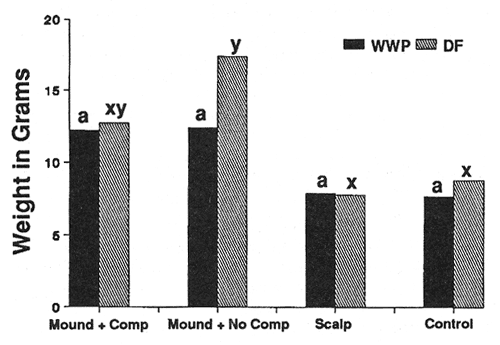
Seedling Weight—Douglas-fir (DF) and western white pine (WWP) responded to site preparation treatments in a similar manner on both sites (figs. 2 and 3). As expected, the heaviest trees occurred on both sites in the mounded treatment that had the competing vegetation removed. Survival was severely limited (P < 0.05) only on the harsh site in mounds with the intense competition left intact (36 percent WWP, 54 percent DF survival after 3 years, compared to at least 76 percent survival for all other site, species, and treatment combinations). Beyond that, however, seedling response was not always as expected. For example, leaving competing vegetation in place on the harsh site in the mounded treatment completely negated the positive effects of reduced bulk density, increased nutrients, organic matter, and moisture (fig. 2). Also, despite the physical removal of competition from both the harsh (fig. 2) and moderate (fig. 3) site scalps, performance in those areas was no better than for the minimal disturbance treatments where competition was left in place.
In general, the pattern of performance that emerged with both species was strong positive responses to the presence and quantity of organic soil components and a strong negative response to competition. Where positive and negative elements were combined, they tended to offset one another.
Figure 2—Total seedling biomass after 3 years (harsh site). Differing letters indicate significant differences (P < 0.05) between treatments. [view larger image - 40K] [Text description of this figure]
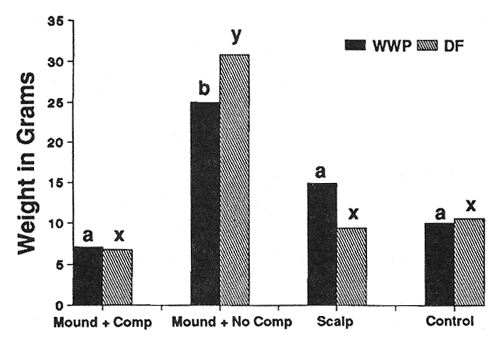
Figure 3—Total seedling biomass after 3 years (moderate site). Differing letters indicate significant differences (P < 0.05) between treatments. [view larger image - 44K] [Text description of this figure]
Ectomycorrhizal Development—Initiation of ectomycorrhizal associations on the root systems of first-year seedlings was rapid on WWP seedlings and relatively slow on DF. Table 1 provides an example. A full complement of ectomycorrhizal short roots (no further significant differences, P = 0.05) was established on the WWP seedlings after only 12 weeks. In contrast, increases in ectomycorrhizae occurred on DF seedlings throughout the full 24-week sampling period in the first season.
| Site | Season | Ectomycorrhizal tips No competition |
Ectomycorrhizal tips Scalp |
|---|---|---|---|
1Different letters indicate significant differences (P < 0.05) between seasons, within species, 16 seedling samples. |
|||
| WWP | Spring | 14.8a | 6.1x |
| WWP | Early summer | 48.5ab | 88.1y |
| WWP | Late summer | 81.7b | 93.2y |
| WWP | Fall | 100.3b | 74.5xy |
| DF | Spring | 0.0a | 0.3x |
| DF | Early summer | .1a | 1.0x |
| DF | Late summer | 7.5a | 21.4x |
| DF | Fall | 25.2b | 45.1y |
Treatment conditions imposed strong controls on the number of ectomycorrhizal short roots (ESR's) supported by individual seedlings of both species (figs. 4 and 5). In general, species responded similarly; significantly higher numbers of ESR's occurred on seedlings in the scalped treatments compared to other treatments. Additionally, the warmer of the two sites (harsh site) generally supported more ESR's per seedling than the middle-elevation (moderate) site for most treatments.
Figure 4—Effect of treatment on numbers of ectomycorrhizal tips per seedling after 3 years (harsh site). Differing letters indicate significant differences (P < 0.05) between treatments. [view larger image - 32K] [Text description of this figure]
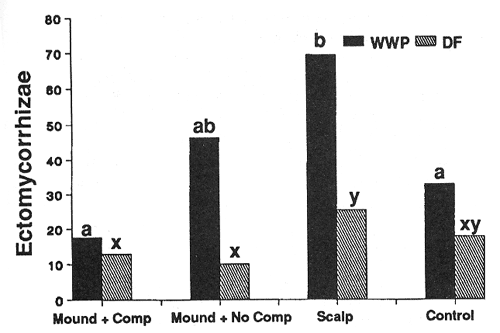
Figure 5—Effect of treatment on numbers of ectomycorrhizal tips per seedling after 3 years (moderate site). Differences among treatments were not significant, differences between species were significant (P < 0.05) on all treatments. [view larger image - 36K] [Text description of this figure]
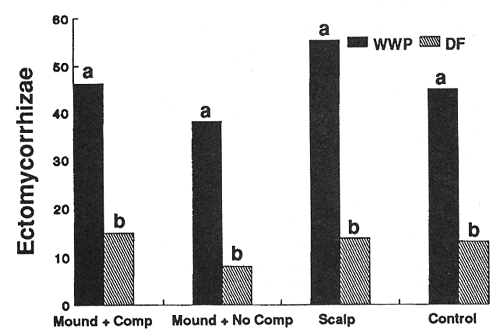
Season (sampling time) also had a strong effect on numbers of ESR's occurring on the seedlings. This effect was most pronounced with WWP seedlings in the mounded treatment with no competing vegetation, and it varied with site (table 2).
| Season | Ectomycorrhizal tips Harsh site |
Ectomycorrhizal tips Moderate site |
|
|---|---|---|---|
1Different letters indicate significant differences (P < 0.05) between seasons, within site, 16 seedling samples. |
|||
| Spring | 152.3a | 61.9x | |
| Early summer | 23.2b | 43.9x | |
| Late summer | 42.8a | 18.6y | |
| Fall | 52.2a | 15.7y | |
Higher numbers of ESR's occurred on WWP than DF seedlings throughout the treatments on either site (figs. 4 and 5, table 3). This relationship was consistent and did not depend on size of root system or seedling top.
In summary, variables within the experiment that influenced numbers of ESR's per seedling included: (1) first year age, (2) treatment, (3) site, and (4) species. No significant differences in ectomycorrhizal morphology were noted when comparing the treatments and variables described here.
| Species | Season | No competition Ectomycorrhizal tips |
No competition Weight (Grams) |
Scalp Ectomycorrhizal tips |
Scalp Weight (Grams) |
|---|---|---|---|---|---|
1Different letters indicate significant differences (P < 0.05) between species at each location, 16 seedling samples. |
|||||
| WWP | Harsh | 160.1a | 25.0a | 98.8x | 14.9x |
| DF | Harsh | 14.6b | 30.8a | 56.4y | 9.4y |
| WWP | Moderate | 55.1a | 12.4a | 65.4x | 7.9x |
| DF | Moderate | 14.8a | 17.4b | 21.9y | 7.8x |
Relationship Between Ectomycorrhizae and Seedling Weight—Not surprisingly, these same variables also affected correlations between the numbers of ESR's supported on seedling root systems and performance of those seedlings (total weight, root weight, or top weight).
Starting with age, tables 4 and 5 show first-year weight was positively correlated, at varying significance levels, with numbers of ESR's across all treatments on both sites. This was the most consistently positive relationship between ectomycorrhizae and growth characteristics that was observed throughout the experiment.
| Harsh site (WWP) |
Year 1 | Year 2 | Year 3 |
|---|---|---|---|
1Sign indicates correlation with growth (+P < 0.1, ++P < 0.05, +++P < 0.01, P < 0.01, N indicates neutral effect), 65 seedling samples. |
|||
| Mound + competition | +1 | ++ | + |
| Mound, no competition | +++ | + | ––– |
| Scalp | + | N | N |
| Control | ++ | + | N |
| Moderate site (DF) |
Year 1 | Year 2 | Year 3 |
|---|---|---|---|
1Sign indicates correlation with growth (+P < 0.1, ++P < 0.05, +++P < 0.01, −−−P < −0.01, N indicates neutral effect), 65 seedling samples. |
|||
| Mound + competition | +++1 | −−− | +++ |
| Mound, no competition | +++ | −−− | N |
| Scalp | +++ | N | N |
| Control | +++ | N | N |
After the first year, correlations were not uniformly positive for all treatments (tables 4 and 5). For example, the relationship between growth and ESR's was often positive only for the mounded treatments with heavy competition. In the presence of heavy competition, positive correlations between ESR's and seedling weight remained relatively consistent, particularly with WWP.
Despite the relatively high numbers of ectomycorrhizal short roots on seedlings of both species in the scalped treatments, on either site with either species, relationships with growth were usually neutral (not significant) in years 2 and 3 (tables 4 and 5). In general, patterns of response between sites were similar but year-to-year variations were noted. For example, the mound treatment (with competition) for DF on the moderate site in year 2 showed a negative rather than a positive correlation (table 5).
Comparing the two species between sites showed strong differential reactions (table 6). For example, when competition and organic matter were high, ectomycorrhizae were positively correlated with seedling weight of both species only on the harsh site. There was a strong negative correlation between ectomycorrhizae and seedling weight of WWP on the moderate site.
| Site (mound + competition) year 3 |
Ectomycorrhizal tips Douglas fir |
Ectomycorrhizal tips Western white pine |
|
|---|---|---|---|
1Sign indicates correlation with growth (+P < 0.1, +++P < 0.01, −P < −0.1), 63 seedling samples. |
|||
| Harsh | 122.2+ | 14.8+ | |
| Moderate | 22.8+++ | 38.3− | |
Seasonal data within year 3 on WWP showed a significant seasonal effect. Both the early and late samples had neutral correlations with growth, but during the midsummer drought there was a positive correlation between numbers of ESR's and seedling weight (table 7).
| Season | Ectomycorrhizal tips year3, scalp, WWP |
||
|---|---|---|---|
1Sign indicates positive correlation with growth (++ P < 0.05, N indicates neutral effect), 26 seedling samples. |
|||
| Spring | 1132.8N | ||
| Early summer | 54.9++ | ||
| Late summer | 117.2++ | ||
| Fall | 58.7N | ||
Combining data for all 3 years from both sites, then comparing species reactions to treatments, showed strong responses between seedling species and individual treatments (table 8). Thus, the same variables that controlled numbers of ESR's per seedling (development) also affected their correlations with growth (function). These variables controlled not only the strength of correlations, but also whether they were positive, negative, or neutral.
| Treatment (all years included) |
Ectomycorrhizal tips Western white pine |
Ectomycorrhizal tips Douglas fir |
|
|---|---|---|---|
1Sign indicates correlation with growth (+P 0.1, −P < −0.1, −−P < −0.05, N indicates neutral effect), 500 seedling samples. |
|||
| Mound + competition | 173.3+ | 38.3−− | |
| Mound + no competition | 43.1N | 21.9−− | |
| Scalp | 64.7− | 40.4−− | |
| Control | 40.5N | 15.8−− | |
We believe these results make it quite evident that the nature of the soil treatment, the site, the season, the species, and time all affect both the ability of seedlings to form ectomycorrhizal associations and the ability of those associations to contribute to seedling growth. Thus, the question regarding where and when ectomycorrhizae provide a favorable return in terms of seedling growth can only be answered when these variables are known. These results are similar to those obtained for endomycorrhizal associations on alpine grasses growing in wildland ecosystems (Fitter 1986).
Perhaps the only exception to this generalization was first-year performance of WWP and DF seedlings growing on these northern Idaho sites. During that year ectomycorrhizae were beneficial to both species for all treatments on both sites (tables 4 and 5). Early establishment of ectomycorrhizal associations appears critical to good performance, even on these relatively moderate sites. This relationship would likely have been even more critical if harsher sites had been used (Amaranthus and Perry 1987).
The relative slowness of DF seedlings to establish a good complement of ESR's during the first season suggests that outplanting seedlings with well-developed ectomycorrhizal associations could improve first-year performance of this species. Amaranthus and Castellano (in preparation) have found significant increases in survival and growth rates of Douglas-fir seedlings inoculated with spores of the ectomycorrhizal fungus Rhizopogon vinicolor on a variety of sites in the Pacific Northwest. However, WWP seedlings responded to the native inoculum on these sites so rapidly that it seems unlikely, except on extremely harsh sites or degraded soils, that routine efforts to produce appropriate ESR's in the nursery could be beneficial. These results also suggest that seral species, with their ability to form ESR's quickly, may be a better choice to reforest many highly disturbed sites with poor soils in northern Idaho than would DF. Other species that fill climax roles in these ecosystems may also be slow to initiate ectomycorrhizal associations.
If, however, seedlings are to be planted on sites where competitive ability would be a major factor in early survival and growth, results of this study suggest that strong mycorrhization of outplanting stock at the nursery would likely be beneficial, even to WWP. Additionally, the ability to positively affect growth in highly competitive situations strongly suggests that effective ectomycorrhizal development may be a key process, not only during early establishment and growth, but also during later stages of succession and, ultimately, in the highly competitive, advanced stages of forest development.
The inability of strong ESR development on both WWP and DF seedlings in scalp treatments to contribute significantly to increased seedling weight indicates that factors limiting growth could not be improved substantially by the presence of active symbionts. It is notable, however, that even with the much higher numbers of ESR's on the smaller seedlings than with other treatments, the correlations between ESR's and growth were not negative as might have been expected. Thus, even with the limited photosynthetic capacity of small seedlings and the large attendant carbon drain brought about by supporting high ESR counts, growth returns to the seedlings were at least sufficient to offset the high relative cost. This, in turn, suggests that the scalp treatment was either lacking in factors needed for growth of the seedlings or it inhibited or delayed the ESR's from acquiring them.
Seasonal relationships between numbers of ESR's and their correlation with growth during midsummer suggest that ectomycorrhizal activities may be particularly important to conifer performance during the intermittent, climatic droughts typical of western-montane forest ecosystems.
Although it is evident from this and other studies that ectomycorrhizal associations can exact a cost to conifer seedlings that is high when compared to the direct returns (increased growth) provided by the association, significant benefits are most often provided in the most critical ecological circumstances: first-year establishment, during drought, and in highly competitive situations. Thus, maintaining ectomycorrhizae on conifer root systems can be viewed as the ecological equivalent of an insurance policy. In many cases the premiums are high when times are good, but in times of crises, benefits may be critical to survival, growth, and competitive ability. Additionally, there are likely to be significant, indirect soil benefits resulting from support of the microbial community in and around conifer root systems (see paper by Molina and Amaranthus, this proceedings).
Alvarez, I. F.; Rowney, D. L.; Cobb, F. W., Jr. 1979. Mycorrhizae and growth of white fir seedlings in mineral soil with and without organic layers in a California forest. Canadian Journal of Forest Research. 9: 311-315.
Amaranthus, M. P.; Castellano, M. A. [In preparation]. Douglas-fir seedling performance following basidiospore inoculation: fungal effectiveness and genotypic variation.
Amaranthus, M. P.; Perry, D. A. 1987. The effect of soil transfer on ectomycorrhiza formation and the survival and growth of conifer seedlings on old, non reforested clear-cuts. Canadian Journal of Forest Research. 17: 944-950.
Amaranthus, M. P.; Trappe, J. M.; Molina, J. 1989. Long-term forest productivity and the living soil. In: Perry, D. A.; Meurisse, R.; Thomas, B.; [and others], eds. Maintaining the long-term productivity of Pacific Northwest ecosystems. Portland, OR: Timber Press: Chapter 3.
Bjorkman, E. 1970. Forest tree mycorrhizaethe conditions for its formation and the significance for tree growth and afforestation. Plant and Soil. 32: 589-610.
Cooper, S. T.; Neiman, K. E.; Steele, R.; Roberts, D. W. 1987. Forest habitat types of northern Idaho: a second approximation. Gen. Tech. Rep. INT-236. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 135 p.
Fitter, A. H. 1986. Effect of benomyl on leaf phosphorus concentration in alpine grasslands: a test of mycorrhizal benefit. New Phytologist. 103: 767-776.
Fogel, R.; Hunt, G. 1983. Contribution of mycorrhizae and soil fungi to nutrient cycling in a Douglas-fir ecosystem. Canadian Journal of Forest Research. 13: 219-232.
Graham, R. T.; Harvey, A. E.; Jurgensen, M. F. 1989a. Effect of site preparation on survival and growth of Douglas-fir (Pseudotsuga menziesii Mirb. Franco.) seedlings. New Forests. 3: 89-98.
Graham, R. T.; Harvey, A. E.; Jurgensen, M. F. 1989b. Site preparation strategies for artificial regeneration: can prescribed burning fill the bill? In: Baumgartner, D. M.; Breuer, D. W.; Zamora, B. A.; Neuenschwander, L. F.; Wakimoto, R. H., eds. Prescribed fire in the intermountain region: symposium proceedings; 1986 March 3-6; Spokane, WA. Pullman, WA: Washington State University, Cooperative Extension: 83-89.
Grier, C. C.; Vogt, K. A.; Keyes, M. R.; Edmonds, R. L. 1981. Biomass distribution and above- and belowground production in young and mature Abies amabilis zone ecosystems of the Washington Cascades. Canadian Journal of Forest Research. 11: 155-167.
Harmon, M. E.; Franklin, J. F.; Swanson, F. J.; [and others]. 1986. Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research. 15: 133-302.
Harvey, A. E. 1982. The importance of residual organic debris in site preparation and amelioration for reforestation. In: Site preparation and fuels management on steep terrain: symposium proceedings; 1982 February 15-17; Spokane, WA. Pullman, WA: Washington State University, Cooperative Extension: 75-85.
Harvey, A. E.; Jurgensen, M. F.; Larsen, M. J. 1976a. Intensive fiber utilization and prescribed fire: effects on the microbial ecology of forests. Gen. Tech. Rep. INT-28. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 46 p.
Harvey, A. E.; Jurgensen, M. F.; Larsen, M. J. 1978. Seasonal distribution of ectomycorrhizae in a mature Douglas-fir/larch forest soil in western Montana. Forest Science. 24: 203-208.
Harvey, A. E.; Larsen, M. J.; Jurgensen, M. F. 1976b. Distribution of ectomycorrhizae in a mature Douglas-fir/larch forest soil in western Montana. Forest Science. 22: 393-398.
Harvey, A. E.; Larsen, M. J.; Jurgensen, M. F. 1979a. Biological implications of increasing harvesting intensity on the maintenance and productivity of forest soils. In: Environmental consequences of timber harvesting in Rocky Mountain coniferous forests. Gen. Tech. Rep. INT-90. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 211-220.
Harvey, A. E.; Larsen, M. J.; Jurgensen, M. F. 1979b. Comparative distribution of ectomycorrhizae in soils of three western Montana forest habitat types. Forest Science. 25: 350-360.
Harvey, A. E.; Jurgensen, M. F.; Larsen, M. J.; Graham, R. T. 1987. Decaying organic materials and soil quality in the Inland Northwest: a management opportunity. Gen. Tech. Rep. INT-225. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 15 p.
Harvey, A. E.; Meurisse, R. T.; Geist, J. M.; Jurgensen, M. F.; McDonald, G. I.; Graham, R. T.; Stark, N. 1989. Managing productivity processes in the Inland Northwest mixed conifers and pines. In: Perry, D. A.; Meurisse, R.; Thomas, B.; [and others], eds. Maintaining the long-term productivity of Pacific Northwest forest ecosystems. Portland, OR: Timber Press: Chapter 9.
Jurgensen, M. F.; Larsen, M. J.; Harvey, A. E. 1979. Forest soil biology-timber harvesting relationships. Gen. Tech. Rep. INT-69. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 12 p.
Krop, B. R.; Castellano, M. A.; Trappe, J. M. 1985. Performance of outplanted western hemlock (Tsuga hetero phylla (Raf.) Sarg.) seedlings inoculated with Cenococcum geophilum. Tree Planters' Notes. Fall: 13-16.
Larsen, M. J.; Harvey, A. E.; Jurgensen, M. F. 1980. Residue decay processes and associated environmental functions in northern Rocky Mountain forests. In: Environmental consequences of timber harvesting in Rocky Mountain coniferous forests. Gen. Tech. Rep. INT-90. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 157-174.
Marshall, J. D.; Perry, D. A. 1987. Basal and maintenance respiration of mycorrhizal and nonmycorrhizal root systems of conifers. Canadian Journal of Forest Research. 17: 872-877.
Maser, C.; Tarrant, R. F.; Trappe, J. M.; Franklin, J. F. 1988. From the forest to the sea: a story of fallen trees. Gen. Tech. Rep. PNW-229. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 153 p.
Maser, C.; Trappe, J. M., eds. 1985. The seen and unseen world of the fallen tree. Gen. Tech. Rep. PNW-164. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 56 p.
McKay, H. M.; Malcolm, D. C. 1988. A comparison of the fine root component of a pure and a mixed coniferous stand. Canadian Journal of Forest Research. 18: 1416-1426.
Miller, S. L.; Durall, D. M.; Rygiewicz, P. T. 1989. Temporal allocation of 14C to extramatrical hyphae of ectomycorrhizal ponderosa pine seedlings. Tree Physiology. 5: 239-249.
Mize, C. W.; Schultz, R. C. 1985. Comparing treatment means correctly and appropriately. Canadian Journal of Forest Research. 16: 1142-1148.
Molina, R. 1982. Use of the ectomycorrhizal fungus Laccaria laccata in forestry. I. Consistency between isolates and effective colonization of containerized conifer seedlings. Canadian Journal of Forest Research. 12: 469-473.
Page-Dumroese, D. S.; Jurgensen, M. F.; Graham, R. T.; Harvey, A. E. 1986. Soil physical properties of raised planting beds in a northern Idaho forest. Res. Pap. INT-360. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 6 p.
Page-Dumroese, D. S.; Jurgensen, M. F.; Graham, R. T.; Harvey, A. E. 1989. Soil chemical properties of raised planting beds in a northern Idaho forest. Res. Pap. INT-419. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 7 p.
Page-Dumroese, D. S.; Loewenstein, H.; Graham, R. T.; Harvey, A. E. 1990. Soil source, seed source and organic matter content effects on Douglas-fir seedling growth. Soil Science Society of America Journal. 54: 229-233.
Perry, D. A.; Amaranthus, M. P.; Borchers, J. G.; Borchers, S. L.; Brainerd, R. E. 1989. Bootstrapping in ecosystems. Bioscience. 39: 230-237.
Perry, D. A.; Molina, R.; Amaranthus, M. P. 1987. Mycorrhizae, mycorrhizospheres, and reforestation: current knowledge and research needs. Canadian Journal of Forest Research. 17: 929-940.
Shaw, C. G., III; Sidle, R. C.; Harris, A. S. 1987. Evaluation of planting sites common to a southeast Alaska clear-cut. III. Effects of microsite type and ectomycorrhizal inoculation on growth and survival of Sitka spruce seedlings. Canadian Journal of Forest Research. 17: 334-339.
Sidle, R. C.; Shaw, C. G., III. 1987. Evaluation of planting sites common to a southeast Alaska clear-cut. IV. Nutrient levels in ectomycorrhizal Sitka spruce seedlings. Canadian Journal of Forest Research. 17: 340-345.
Slankis, V. 1974. Soil factors influencing formation of ectomycorrhizae. Annual Review of Phytopathology. 12: 437-457.
Steele, R. D.; Torrie, J. H. 1960. Principles and procedures of statistics. New York: McGraw-Hill. 481 p.
St. John, T. V.; Coleman, D. C. 1983. The role of mycorrhizae in plant ecology. Canadian Journal of Botany. 61: 1005-1014.
Trappe, J. M.; Strand, R. F. 1969. Mycorrhizal deficiency in a Douglas-fir region nursery. Forest Science. 15: 381-389.
Vogt, K. A.; Edmonds, R. L.; Grier, C. C. 1981. Seasonal changes in biomass and vertical distribution of mycorrhizal and fibrous-textured conifer fine roots in 23- and 180-year-old subalpine Abies amabilis stands. Canadian Journal of Forest Research. 11: 223-229.
Vogt, K. A.; Grier, C. C.; Meier, C. E.; Edmonds, R. L. 1982. Mycorrhizal role in net primary production and nutrient cycling in Abies amabilis ecosystems in western Washington. Ecology. 63: 370-380.
Vogt, K. A.; Moore, E. E.; Vogt, D. J.; Redlin, M. J.; Edmonds, R. L. 1983. Conifer fine root and mycorrhizal root biomass within the forest floors of Douglas-fir stands of different ages and site productivities. Canadian Journal of Forest Research. 13: 429-437.
Speakers answered questions from the audience after their presentations. Following are the questions and answers on this topic:
Q. What is the function of decaying roots in maintaining soil productivity? Do roots compensate for excessive organic matter removal from the soil surface?
A. Decaying roots have important positive functions the same as any other buried woody materials, with the added advantage that they are somewhat protected from loss due to fire. In some situations decaying roots could compensate for loss of surface wood; however, in many inland ecosystems the volume of root material would be well short of the woody debris requirement and it might not replace forest floor horizons adequately because it is buried.
Q. Are ectomycorrhizae important on dry ponderosa pine sites?
A. Yes, they are fully as important as on any other site, perhaps more so because of the relative harshness of dry ecosystems.
Q. Why was establishment of the ectomycorrhizal complement of Douglas-fir so slow compared to that of western white pine?
A. We hypothesize that this is at least in part a genetically programmed trait. We assume that rapid formation of ectomycorrhizae is especially critical to species like the various pines that usually fulfill pioneer and early seral roles in these ecosystems. Thus, they would normally be faced with disturbed, possibly degraded soils and harsh environments.
Q. Have you evaluated qualitative as well as quantitative aspects of ectomycorrhizal behavior in inland ecosystems?
A. Yes. We have noted some differences in ectomycorrhizal types in various circumstances; for example, in general, the harsher the ecosystem or site the more types we are likely to see. However, the variation has usually been so high that specific differences are not supportable statistically.
Paper presented at the Symposium on Management and Productivity of Western-Montane Forest Soils, Boise, ID, April 10-12, 1990.
Alan E. Harvey, Deborah S. Page-Dumroese, and Russell T. Graham are Project Leader, Research Soil Scientist, and Research Forester, respectively, with the Intermountain Research Station, Forest Service, U.S. Department of Agriculture, Moscow, ID 83843. Martin F. Jurgensen is Professor of Forest Soils, School of Forestry and Wood Products, Michigan Technological University, Houghton, MI 49931.